Aardvark Therapeutics Announces ARD-201 Preclinical Obesity Data Showing Significant Weight Loss as a Monotherapy, Enhancement of GLP-1RA Therapy in Combination, and Effective Maintenance Following Discontinuation of GLP-1RA Therapy
Rhea-AI Summary
Aardvark Therapeutics (NASDAQ:AARD) has announced promising preclinical data for ARD-201, its novel obesity treatment candidate. In diet-induced obesity (DIO) mouse studies, ARD-201 demonstrated 19% body weight reduction after 30 days as a monotherapy. The oral treatment showed significant potential in three key areas: preventing weight regain after GLP-1RA discontinuation, standalone weight loss therapy, and enhanced efficacy when combined with low-dose tirzepatide.
The company plans to advance ARD-201 into two Phase 2 trials: The POWER trial (2H 2025) will evaluate weight maintenance after GLP-1RA discontinuation, while the STRENGTH trial (1H 2026) will assess ARD-201's effectiveness alone and in combination with GLP-1RA therapy. These trials replace the previously planned EMPOWER trial.
Positive
- Significant weight loss of 19% achieved with ARD-201 monotherapy in preclinical studies
- ARD-201 demonstrated effectiveness in maintaining weight loss after GLP-1RA discontinuation
- Combination with low-dose tirzepatide showed superior results compared to high-dose tirzepatide alone
- Oral administration offers potential advantage over injectable alternatives
Negative
- Results are only preclinical - no human trial data yet
- Phase 2 trials won't begin until late 2025/early 2026
- Previous EMPOWER trial plans cancelled in favor of new trial design
Insights
ARD-201 shows promising preclinical obesity data with 19% weight loss and potential to complement or follow GLP-1 therapies.
Aardvark Therapeutics has revealed compelling preclinical data for ARD-201, positioning it as a potential triple-threat in obesity management. In the diet-induced obesity (DIO) mouse model - considered highly predictive for GLP-1 therapies - ARD-201 demonstrated ~19% weight reduction as a monotherapy within 30 days, comparable to leading injectable treatments but in an oral formulation.
What's particularly noteworthy is ARD-201's novel application in post-GLP-1RA therapy. The data suggests it can effectively prevent weight rebound after patients discontinue tirzepatide treatment - addressing a critical unmet need in obesity management where patients typically regain weight after stopping treatment. This could potentially allow patients an "exit strategy" from expensive injectable therapies while maintaining benefits.
The combination therapy findings are equally significant. When paired with low-dose tirzepatide (1 nmol/kg/day), ARD-201 produced superior weight loss compared to high-dose tirzepatide (10 nmol/kg/day) alone. This suggests a potential dose-sparing effect that could improve tolerability while maintaining or enhancing efficacy.
The company's strategic pivot to two focused Phase 2 trials (POWER and STRENGTH) instead of the previously planned EMPOWER trial demonstrates clinical development sophistication. The POWER trial addressing weight maintenance after GLP-1RA discontinuation targets a significant market gap, while the STRENGTH trial will assess ARD-201's standalone efficacy and synergistic potential.
While these are preclinical results, the validation from a leading obesity researcher (Dr. Randy Seeley) and the use of a gold-standard animal model known for translational relevance to human outcomes strengthen the clinical potential of these findings.
- In a gold-standard model of obesity, treatment with ARD-201 resulted in approximately
19% body weight reduction after 30 days of treatment. - ARD-201 was also associated with attenuated weight regain after discontinuation of tirzepatide.
- In combination with low-dose tirzepatide, ARD-201 improved weight loss compared to high dose tirzepatide alone.
- Aardvark plans two separate Phase 2 clinical trials for ARD-201: Phase 2 POWER trial to focus on weight rebound in patients discontinuing GLP-1RA therapy, and Phase 2 STRENGTH trial to focus on weight loss as a monotherapy and in combination with GLP-1RA.
SAN DIEGO, Aug. 12, 2025 (GLOBE NEWSWIRE) -- Aardvark Therapeutics, Inc. (Aardvark) (Nasdaq: AARD), a clinical-stage biopharmaceutical company focused on developing novel, small-molecule therapeutics to activate innate homeostatic pathways for the treatment of metabolic diseases, today announced new positive preclinical data demonstrating the potential of ARD-201 for the treatment of metabolic obesity and obesity-related conditions. Data in the validated diet-induced obesity (DIO) mouse model (a conventional model recognized for its strong translational relevance in the field of glucagon-like peptide-1 receptor agonists (GLP-1RAs)) demonstrated potential applications for ARD-201, including for the attenuation of weight gain after withdrawal from GLP-1RA therapies, as a monotherapy for weight loss without GLP-1RA therapy, as well as for weight loss in combination with GLP-1RA therapy. Details of the study will be submitted for peer review publication.
“Weight maintenance remains a major challenge for many patients seeking an off-ramp from treatment with GLP-1RA therapies. These new findings highlight the potential of ARD-201 as an oral therapy that can help patients to not only achieve meaningful weight loss but also to sustain it,” said Timothy Kieffer, Ph.D., Chief Scientific Officer at Aardvark. “The low dose of tirzepatide corresponds to a human equivalent dose substantially lower than what is used clinically, supporting the potential for improved tolerability with a highly complementary oral option.”
In a study designed to show the potential of ARD-201 as a weight loss therapy, DIO mice treated with oral ARD-201 showed substantial weight loss of approximately
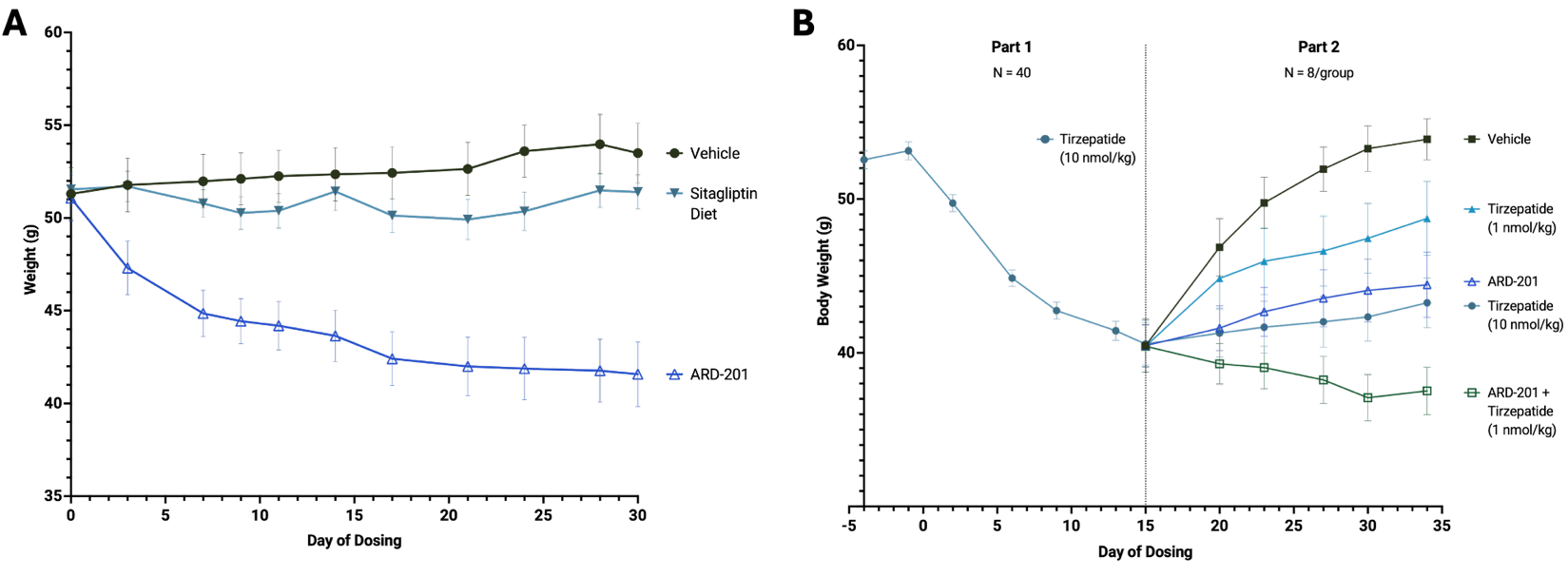
(A) Body weight changes in DIO mice treated with ARD-201, sitagliptin diet, or vehicle.
(B) Body weight in mice given a high-dose of tirzepatide (10 nmol/kg/day, Part 1) followed by either low-dose tirzepatide (1 nmol/kg/day), high-dose tirzepatide (10 nmol/kg/day), ARD-201, or ARD-201-tirzepatide combo (Part 2).
Randy J. Seeley, Henry King Ransom Professor of Surgery at the University of Michigan, who was not associated with the study, commented: "Innovation in obesity therapy will come in different ways. Aardvark is harnessing a unique mechanism that has the potential to produce significant weight loss on its own in pill form and be used in combination with a GLP-1 agonist for additional benefits. Using compounds uniquely in the weight maintenance phase opens up the real possibility of using different approaches to induce weight loss, and then help patients keep that weight off for the long run.”
Driven by these new preclinical insights, Aardvark is advancing ARD-201 into two Phase 2 trials:
Phase 2 POWER Trial (Prevention Of WEight Regain):
- Expected to initiate in 2H 2025, this trial will evaluate ARD-201’s potential to prevent weight regain in subjects who discontinue GLP-1RA therapy after achieving substantial prior weight loss (~
15% ), reducing the burden of chronic injectable use. - The trial will assess outcomes over 24 weeks with an interim analysis at 12 weeks.
Phase 2 STRENGTH Trial (Sitagliptin and TAS2R for weight Reduction with Exercise, Nutrition, and GLP-1RA Trial and Hunger assessment):
- Planned for initiation in 1H 2026, this trial will explore placebo-adjusted weight loss of ARD-201 alone and the additive effects of ARD-201 combined with GLP-1RA therapy.
- Key secondary endpoints include assessing absolute weight loss and the quality of weight loss, specifically evaluating lean muscle versus fat reduction.
These two focused trials will replace the previously planned EMPOWER trial and are designed to enhance the precision and clarity of data collection compared to the EMPOWER trial.
“These results from a validated, predictive preclinical model strengthen our conviction in the ARD-201 program," said Tien Lee, M.D., Founder and Chief Executive Officer of Aardvark. “ARD-201’s oral, combination approach could alter the obesity treatment landscape by providing a new solution for weight maintenance after successful GLP-1 therapy.”
About ARD-201
ARD-201 is an oral fixed dose combination of a dipeptidyl peptidase4 (DPP4) inhibitor and ARD-101, a gut restricted small molecule agonist of select taste receptors (TAS2Rs) expressed in the intestinal lumen. These receptors normally respond to nutrients and are part of the gut brain axis that helps regulate food intake. Activation of TAS2Rs stimulates the release of endogenous signaling molecules, including cholecystokinin (CCK) and GLP-1, which play key roles in promoting satiety and reducing hunger. DPP4 inhibitors, which are widely used for the treatment of diabetes, extend the biological activity of gut hormones, including GLP-1, by preventing their enzymatic inactivation. Together, these mechanisms allow ARD-201 to enhance and prolong the body’s natural signals for fullness.
Separately, ARD-101 is being evaluated in the Phase 3 HERO (Hunger Elimination or Reduction Objective) trial for hyperphagia associated with Prader-Willi Syndrome (PWS).
About Aardvark Therapeutics, Inc.
Aardvark is a clinical-stage biopharmaceutical company developing novel, small-molecule therapeutics designed to suppress hunger for the treatment of Prader-Willi Syndrome (PWS) and metabolic diseases. As we recognize that hunger (the discomfort from not having eaten recently) is a distinct neural signaling pathway separate from appetite (the reward-seeking, desirability of food), our programs are designed to explore therapeutic applications in hunger-associated indications and potential complementary uses with anti-appetite therapies. Our lead compound, oral ARD-101, is in Phase 3 clinical development for the treatment of hyperphagia associated with PWS, a rare disease characterized by insatiable hunger. ARD-101 is also being studied in hypothalamic obesity. Additionally, Aardvark is developing ARD-201, a fixed-dose combination of ARD-101 with a DPP-4 inhibitor, and conducting two separate trials, with a goal of addressing some of the limitations of currently marketed GLP-1RA therapies for the treatment of obesity and obesity-related conditions. For more information, visit aardvarktherapeutics.com.
Forward-Looking Statements
Statements in this press release about future expectations, plans and prospects, as well as any other statements regarding matters that are not historical facts, may constitute “forward-looking statements.” These statements include, but are not limited to, statements concerning: Aardvark’s future results of operations and financial position, business strategy, product candidates, ongoing clinical trials, planned clinical trials, expected timing for data readouts and reporting topline results, anticipated cash runway, likelihood of success, as well as plans and objectives of management for future operations. Words including, without limitation, “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these or similar identifying words. Forward-looking statements in this press release include statements regarding ARD-201’s potential, including its potential as an oral therapy that can help patients achieve meaningful weight loss and also help to sustain it; the potential for ARD-201 to provide improved tolerability or to be a highly complementary oral option; the potential for ARD-201 to alter the obesity treatment landscape or provide a new solution for weight maintenance after successful GLP-1 therapy; the trial design for the POWER and STRENGTH trials and the expected timing for commencing such trials; and the development path for ARD-201. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including: uncertainties related to potential delays in the commencement, enrollment and completion of clinical trials; the risk that we may use our capital resources sooner than expected and that they may be insufficient to allow us to achieve our anticipated milestones; risks related to our dependence on third parties for manufacturing, shipping and production of drug product for use in clinical and preclinical trials; the risk of unfavorable clinical trial results; the risk that results from earlier clinical trials and preclinical studies may not necessarily be predictive of future results; and other factors discussed in the “Risk Factors” section of Aardvark’s most recent Annual Report on Form 10-K and Quarterly Reports on Form 10-Q that Aardvark has filed or may subsequently file with the U.S. Securities and Exchange Commission. When evaluating Aardvark’s business and prospects, careful consideration should be given to these risks and uncertainties. Any forward-looking statements contained in this press release are based on the current expectations of Aardvark’s management team and speak only as of the date hereof, and Aardvark specifically disclaims any obligation to update any forward-looking statement, whether as a result of new information, future events or otherwise.
Contact:
Carolyn Hawley, Inizio Evoke Comms
(619) 849-5382
Carolyn.hawley@inizioevoke.com








