Aprea Reports Anti-Proliferative Results and Promising Early-Stage Clinical Data for Next-Generation WEE1 Inhibitor, APR-1051, in HPV+ Head and Neck Squamous Cell Carcinoma (HNSCC) in Collaboration with MD Anderson Cancer Center
Rhea-AI Summary
Aprea Therapeutics (NASDAQ:APRE) has announced promising preclinical and early clinical data for APR-1051, its next-generation oral WEE1 inhibitor, in treating HPV+ head and neck squamous cell carcinoma (HNSCC). The drug demonstrated potent antiproliferative effects with IC₅₀ values between 8.9-230 nM in preclinical studies.
In collaboration with MD Anderson Cancer Center, research showed significant anti-tumor synergy when combining APR-1051 with anti-PD-1 therapies. In the Phase 1 ACESOT-1051 trial, a 62-year-old male patient with advanced HPV+ cancer showed stable disease with 5% tumor reduction at first assessment, with no dose-limiting toxicities reported.
Positive
- None.
Negative
- Initial clinical data limited to only one patient
- Current dosing at subtherapeutic 70mg level
- Still in early Phase 1 stage of development
News Market Reaction
On the day this news was published, APRE gained 10.09%, reflecting a significant positive market reaction.
Data tracked by StockTitan Argus on the day of publication.
Preclinical data demonstrate potent single-agent and combination effects in head and neck squamous cell carcinoma (HNSCC) models, including synergy with anti–PD-1 therapy
Initial Phase 1 clinical update shows early disease control in first HPV+ patient treated with APR-1051
DOYLESTOWN, Pa., June 25, 2025 (GLOBE NEWSWIRE) -- Aprea Therapeutics, Inc. (Nasdaq: APRE) (“Aprea”, or the “Company”), a clinical-stage biopharmaceutical company developing innovative treatments that exploit specific cancer cell vulnerabilities while minimizing damage to healthy cells, today announced new preclinical data and a clinical update on APR-1051, the Company’s next-generation oral WEE1 inhibitor, in human papillomavirus–positive (HPV+) head and neck squamous cell carcinoma (HNSCC).
These findings result from an ongoing translational research collaboration with renowned oncology leader MD Anderson Cancer Center and support the potential of APR-1051 both as a single agent and in rational immunotherapy combinations for biomarker-driven treatment of HPV+ HNSCC. “We are excited by the preclinical data generated by independent researchers, and the early clinical signal of APR-1051 in an HPV-positive cancer patient,” said Oren Gilad, Ph.D., President and Chief Executive Officer of Aprea Therapeutics. “We believe that APR-1051 could offer significant differentiation in the competitive oncology landscape, as a single agent, as well as in combination with checkpoint inhibitors.”
Preclinical Highlights from MD Anderson Collaboration:
- Potent single-agent activity: APR-1051 demonstrated robust antiproliferative effects across a broad panel of human and murine head and neck cancer cell lines, including HPV+ subtypes, with IC₅₀ values ranging from 8.9 to 230 nM.
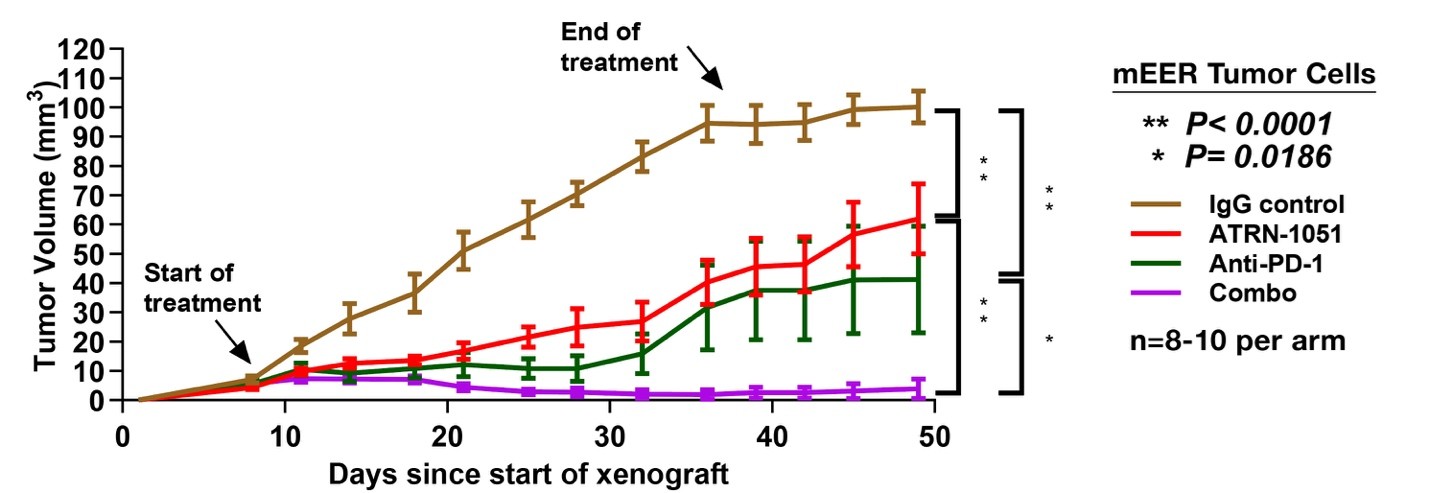
- Enhanced combination synergy: Significant anti-tumor synergy was observed with APR-1051 and anti–PD-1 therapies in HPV+ HNSCC models, positioning APR-1051 as a candidate for combination-based clinical trials.
- Mechanistic rationale: APR-1051 was shown to activate cGAS/STING-mediated immunogenic cell death and to exploit the HPV E6-driven G2 checkpoint dependency in HPV+ tumors. Given WEE1’s central role in regulating the G2/M checkpoint, HPV+ tumor cells appear highly reliant on WEE1 signaling for survival. This provides a biomarker driven strategy for targeted patient selection and optimized clinical outcomes.
The chart below shows how APR-1051 potentiatiated the immune response to checkpoint inhibitors in an HPV+ HNSCC model.
Clinical Update from Phase 1 ACESOT‑1051 Trial:
- In a 62-year-old male with advanced HPV-positive oropharyngeal squamous cell carcinoma who had progressed after three prior lines of platinum-based therapy, once-daily administration of a subtherapeutic 70 mg oral dose of APR-1051 resulted in stable disease with a
5% tumor reduction at the first radiographic assessment. - The patient tolerated therapy well, with no dose-limiting toxicities reported.
Next Steps and Future Development:
- Enrollment in the ACESOT-1051 trial is ongoing and progressing, with dose escalation into higher levels and the continued inclusion of HPV+ patients.
- Pending additional data, future trial arms may evaluate APR-1051 in combination with checkpoint inhibitors to address unmet medical needs across distinct patient populations.
Drs. Abdullah Osman and Jeffrey Myers from The University of Texas MD Anderson Cancer Center commented, "We are very encouraged by these early findings and see APR-1051 as a potentially promising addition to the therapeutic portfolio for treating HPV-associated head and neck cancers. The mechanistic rationale and robust preclinical data strongly support the potential for enhanced patient outcomes when APR-1051 is administrated as a single agent or in combination with existing immunotherapies."
Aprea remains committed to advancing APR-1051 as a next-generation precision oncology agent in molecularly defined tumors, leveraging biomarker insights to optimize patient outcomes.
About APR-1051
APR-1051 is an oral, highly selective WEE1 inhibitor designed to minimize off-target activity and optimize pharmacologic selectivity. APR-1051 is currently being evaluated in the ACESOT-1051 Phase 1 clinical trial (NCT06260514) in patients with advanced solid tumors harboring DNA damage response (DDR) alterations.
About Aprea
Aprea is pioneering a new approach to treat cancer by exploiting vulnerabilities associated with cancer cell mutations. This approach was developed to kill tumors but to minimize the effect on normal, healthy cells, decreasing the risk of toxicity that is frequently associated with chemotherapy and other treatments. Aprea’s technology has potential applications across multiple cancer types, enabling it to target a range of tumors, including ovarian, colorectal, prostate, and breast cancers. The company’s lead programs are APR-1051, an oral, small-molecule inhibitor of WEE1 kinase, and ATRN-119, a small molecule ATR inhibitor, both in clinical development for solid tumor indications. For more information, please visit the company website at www.aprea.com
The Company may use, and intends to use, its investor relations website at https://ir.aprea.com/ as a means of disclosing material nonpublic information and for complying with its disclosure obligations under Regulation FD.
Forward-Looking Statement
Certain information contained in this press release includes “forward-looking statements”, within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended related to our study analyses, clinical trials, regulatory submissions, and projected cash position. We may, in some cases use terms such as “future,” “predicts,” “believes,” “potential,” “continue,” “anticipates,” “estimates,” “expects,” “plans,” “intends,” “targeting,” “confidence,” “may,” “could,” “might,” “likely,” “will,” “should” or other words that convey uncertainty of the future events or outcomes to identify these forward-looking statements. Our forward-looking statements are based on current beliefs and expectations of our management team and on information currently available to management that involve risks, potential changes in circumstances, assumptions, and uncertainties. All statements contained in this press release other than statements of historical fact are forward-looking statements, including statements regarding our ability to develop, commercialize, and achieve market acceptance of our current and planned products and services, our research and development efforts, including timing considerations and other matters regarding our business strategies, use of capital, results of operations and financial position, and plans and objectives for future operations. Any or all of the forward-looking statements may turn out to be wrong or be affected by inaccurate assumptions we might make or by known or unknown risks and uncertainties. These forward-looking statements are subject to risks and uncertainties including, without limitation, risks related to the success, timing, and cost of our ongoing clinical trials and anticipated clinical trials for our current product candidates, including statements regarding the timing of initiation, pace of enrollment and completion of the trials (including our ability to fully fund our disclosed clinical trials, which assumes no material changes to our currently projected expenses), futility analyses, presentations at conferences and data reported in an abstract, and receipt of interim or preliminary results (including, without limitation, any preclinical results or data), which are not necessarily indicative of the final results of our ongoing clinical trials, our understanding of product candidates mechanisms of action and interpretation of preclinical and early clinical results from its clinical development programs, our ability to continue as a going concern, and the other risks, uncertainties, and other factors described under “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and elsewhere in the documents we file with the U.S. Securities and Exchange Commission. For all these reasons, actual results and developments could be materially different from those expressed in or implied by our forward-looking statements. You are cautioned not to place undue reliance on these forward-looking statements, which are made only as of the date of this press release. We undertake no obligation to update such forward-looking statements for any reason, except as required by law.
Investor Contact:
Mike Moyer
LifeSci Advisors
mmoyer@lifesciadvisors.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/d8dcbce3-58cc-4c7f-af86-00f1440e02b4









