Aspire Biopharma's Unveils Bold New Era for BUZZ BOMB(TM) Caffeine with Dynamic Website and Packaging Redesign
Rhea-AI Summary
Aspire Biopharma (NASDAQ:ASBP) announced a comprehensive brand redesign for its flagship caffeine product, BUZZ BOMB™ on January 21, 2026. The initiative includes a new logo, a mobile‑optimized e-commerce website at https://buzzbombcaffeine.com, and redesigned packaging with a vibrant aesthetic to emphasize rapid, sustained focus and sublingual delivery.
The website offers a streamlined shopping experience, product information, promotions, and educational content. Management says the redesign aims to improve customer engagement, showcase the product's capabilities, and position BUZZ BOMB for growth.
Positive
- None.
Negative
- None.
News Market Reaction
On the day this news was published, ASBP declined 4.98%, reflecting a moderate negative market reaction. Argus tracked a peak move of +11.9% during that session. Argus tracked a trough of -14.9% from its starting point during tracking. Our momentum scanner triggered 15 alerts that day, indicating notable trading interest and price volatility. This price movement removed approximately $269K from the company's valuation, bringing the market cap to $5M at that time.
Data tracked by StockTitan Argus on the day of publication.
Market Reality Check
Peers on Argus
ASBP fell 9.05% while peers were mixed: QTTB up 13.26%, NRXS up 2.65%, and ICU down 8.43%. With no peers in the momentum scanner and divergent moves, trading appears more stock-specific than sector-driven.
Historical Context
| Date | Event | Sentiment | Move | Catalyst |
|---|---|---|---|---|
| Jan 14 | Reverse stock split | Negative | -26.4% | 1-for-40 reverse split to address Nasdaq bid-price deficiency. |
| Jan 07 | Regulatory update | Positive | +19.6% | FDA pre-IND feedback supports 505(b)(2) NDA path for OTASA. |
| Dec 18 | Marketing partnership | Positive | +4.6% | Elite ultra-runner appointed global BUZZ BOMB brand ambassador. |
| Dec 12 | Nasdaq extension | Negative | -9.1% | Nasdaq grants limited time to regain bid-price and equity compliance. |
| Dec 04 | Business update | Neutral | +2.5% | Q3 update on aspirin program, IP filings, and BUZZ BOMB production. |
Price moves have generally aligned with news tone: regulatory and financing overhangs saw declines, while clinical and commercial milestones produced gains.
Over the last two months, Aspire Biopharma has balanced clinical and commercial progress with listing and capital-structure pressures. A 1-for-40 reverse split on Jan 16, 2026 and Nasdaq compliance extensions framed significant dilution and listing risk, both followed by share-price declines. In contrast, positive regulatory steps for its sublingual aspirin program and BUZZ BOMB™ commercialization (including a Q3 2025 business update and a high-profile brand ambassador on Dec 18, 2025) coincided with modest gains. Today’s BUZZ BOMB™ rebranding fits the ongoing push to build its consumer caffeine franchise.
Market Pulse Summary
This announcement highlights Aspire’s continued investment in BUZZ BOMB™ through a new website and packaging meant to improve user engagement and brand visibility. It complements earlier steps like the Dec 18, 2025 brand ambassador deal and Q3 2025 production ramp. At the same time, SEC filings describe tight liquidity, significant losses, and Nasdaq compliance obligations. Investors may watch how this rebranding impacts BUZZ BOMB™ sales alongside progress on the sublingual aspirin regulatory pathway.
Key Terms
sublingual delivery medical
e-commerce technical
mobile-optimized technical
AI-generated analysis. Not financial advice.
New streamlined, mobile-optimized shopping experience, allows consumers to purchase BUZZ BOMB™ directly, access exclusive promotions and engage with the brand through educational and lifestyle content highlighting the benefits of sublingual delivery
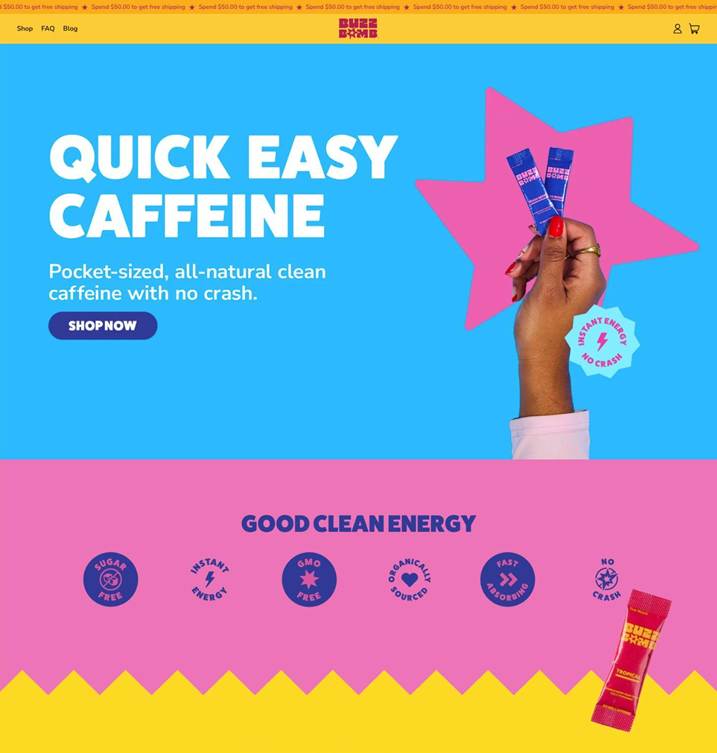
ESTERO, FL / ACCESS Newswire / January 21, 2026 / Aspire Biopharma Holdings, Inc. (NASDAQ:ASBP) ("Aspire" or the "Company"), a biopharmaceutical company developing multi-faceted patent-pending delivery technology, is proud to announce the launch of a comprehensive brand redesign for its flagship caffeine product, BUZZ BOMB™. This strategic rebranding initiative introduces a new logo, sleek, modern website and eye-catching product packaging, reflecting the product's capabilities and Aspire Biopharma's commitment to cutting-edge science.
Enhanced Digital Experience
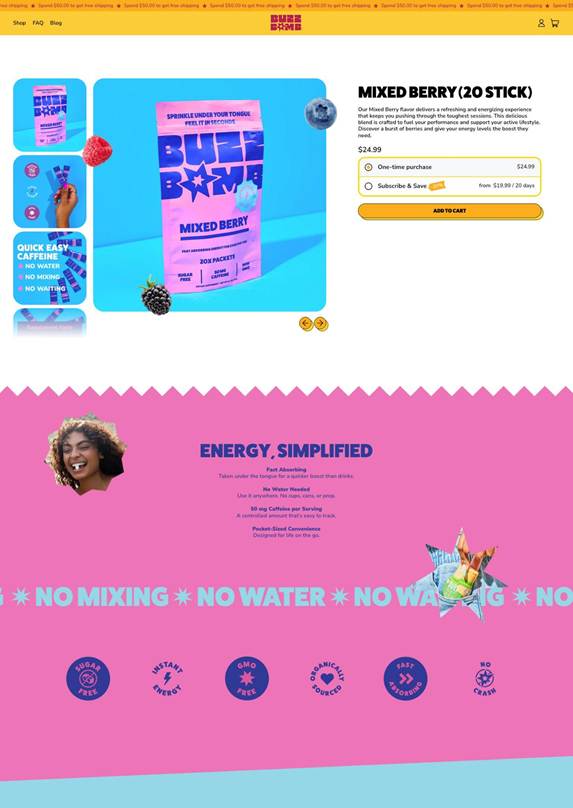
The new BUZZ BOMB™ website, now live at https://buzzbombcaffeine.com, offers a seamless, user-friendly experience ensuring customers can quickly and efficiently access their favorite energy solution. This upgraded shopping experience provides detailed product information, and an intuitive e-commerce interface, bringing a user-friendly, modern experience to consumers looking to bring energy back into their everyday lives.
New BUZZ BOMB™ E-Commerce Website Landing Page
Bold New Packaging
The newly redesigned packaging features a modern bold aesthetic, utilizing a vibrant color palette and bold typography that visually communicate immediate impact and efficacy. This refreshed look is designed to stand out and clearly convey the product's promise of rapid, sustained focus and energy for peak performance not only for athletes, but the everyday person looking for a boost. It's modern and the future of everyday caffeine.
BUZZ BOMB™ Newly Designed Packaging
"Our goal was to create a visual identity that matches the power of the product inside," said Kraig Higginson, Interim CEO of Aspire. "We are committed to providing our customers with carefully formulated products that deliver real results. We worked with Thistle Marketing and our brand team to completely reconceptualize our BUZZ BOMB™ brand identity. The new design elements perfectly capture the intensity and precision of the product experience, and our new website offers an elevated platform to connect with our audience and share our story."
BUZZ BOMB™ Mixed Berry Packets on ordering page
BUZZ BOMB™ is committed to developing unique caffeine products that deliver immediate, reliable energy without compromise. The new aesthetic and digital experience underscore BUZZ BOMB™'s commitment to innovation and customer satisfaction, positioning the brand for significant new growth potential.
About BUZZ BOMB™
BUZZ BOMB™ is a proprietary sublingual caffeine supplement developed by Aspire Biopharma that features 50mg of caffeine and is currently offered in four flavors (Tropical Fruit, Mixed Berry, Peach Mango, and Coffee Mocha). Designed for athletes, professionals, and the everyday person needing a rapid cognitive boost, BUZZ BOMB® provides a precise dose of caffeine that bypasses the GI tract for faster onset and smoother energy. To learn more about BUZZ BOMB™, or purchase product online, please visit https://buzzbombcaffeine.com.
About Aspire Biopharma Holdings, Inc.
Aspire Biopharma has developed a patent-pending sublingual delivery technology that can deliver drugs to the body rapidly and precisely. This technology offers the potential to improve effectiveness and reduce side effects by going directly to the bloodstream and avoiding the gastrointestinal tract. Aspire Biopharma's delivery technology can be applied to many different active pharmaceutical ingredients (APIs) and other bioactive substances, spanning both small and large molecule therapeutics, nutraceuticals and supplements.
For more information, please visit www.aspirebiolabs.com
Aspire Biopharma Holdings, Inc.
Contact
PCG Advisory
Kevin McGrath
+1-646-418-7002
kevin@pcgadvisory.com
Safe Harbor Statement
This press release contains "forward-looking statements" within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended, which are intended to be covered by the "safe harbor" provisions created by those laws. Aspire's forward-looking statements include, but are not limited to, statements regarding our or our management team's expectations, hopes, beliefs, intentions or strategies regarding our future operations. In addition, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. The words "anticipate," "believe," "contemplate," "continue," "estimate," "expect," "intends," "may," "might," "plan," "possible," "potential," "predict," "project," "should," "will," "would," and similar expressions may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking. These forward-looking statements represent our views as of the date of this press release and involve a number of judgments, risks and uncertainties. We anticipate that subsequent events and developments will cause our views to change. We undertake no obligation to update forward-looking statements to reflect events or circumstances after the date they were made, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws. Accordingly, forward-looking statements should not be relied upon as representing our views as of any subsequent date. As a result of a number of known and unknown risks and uncertainties, our actual results or performance may be materially different from those expressed or implied by these forward-looking statements. Some factors that could cause actual results to differ include general market conditions, whether clinical trials demonstrate the efficacy and safety of our drug candidates to the satisfaction of regulatory authorities, or do not otherwise produce positive results which may cause us to incur additional costs or experience delays in completing, or ultimately be unable to complete the development and commercialization of our drug candidates; the clinical results for our drug candidates, which may not support further development or marketing approval; actions of regulatory agencies, which may affect the initiation, timing and progress of clinical trials and marketing approval; our ability to achieve commercial success for our drug candidates, if approved; Aspire's acetylsalicylic acid sublingual powder, 162 mg (OTASA) is an investigational new drug and has not been approved for marketing for any indication, our limited operating history and our ability to obtain additional funding for operations and to complete the development and commercialization of our drug candidates; that the Company will be able to meet the deadlines or conditions imposed by the Hearings Panel or regain compliance with all applicable requirements for continued listing, and other risks and uncertainties set forth in "Risk Factors" in our most recent Annual Report on Form 10-K and any subsequent Quarterly Reports on Form 10-Q. In addition, statements that "we believe" and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this press release, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain, and you are cautioned not to rely unduly upon these statements. All information in this press release is as of the date of this press release. The information contained in any website referenced herein is not, and shall not be deemed to be, part of or incorporated into this press release.
SOURCE: Aspire Biopharma Holdings, Inc.
View the original press release on ACCESS Newswire