Asensus Surgical Receives FDA 510(k) Clearance for Senhance Surgical System in Urology
Rhea-AI Summary
Asensus Surgical (NYSE American: ASXC) has received FDA 510(k) clearance for an expanded indication to treat adult and pediatric Urology patients with the Senhance® Surgical System in the United States. This clearance opens up a significant market, with over 185,000 urological surgical procedures performed annually in the U.S. The Senhance System offers precision and advanced digital capabilities, including 3mm and 5mm instruments and digital integrations, making it well-suited for urological procedures.
The system aims to increase surgeon control and reduce variability through Augmented Intelligence and deep learning capabilities. This expansion builds on the system's successful use in Urology procedures outside the U.S. for several years, now bringing its benefits to the U.S. patient population.
Positive
- FDA 510(k) clearance received for expanded indication in Urology
- Access to a market of over 185,000 annual urological procedures in the U.S.
- Senhance System offers precision and advanced digital capabilities suited for urological procedures
- System includes 3mm and 5mm instruments and digital integrations
- Potential to increase surgeon control and reduce variability through AI and deep learning
Negative
- None.
News Market Reaction – ASXC
On the day this news was published, ASXC declined 0.18%, reflecting a mild negative market reaction.
Data tracked by StockTitan Argus on the day of publication.
RESEARCH TRIANGLE PARK, N.C., July 23, 2024 (GLOBE NEWSWIRE) -- Asensus Surgical, Inc. (NYSE American: ASXC), a global leader of innovative digital solutions for the operating room, today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (“FDA”) for an expanded indication to treat adult and pediatric Urology patients with the Senhance® Surgical System.
“This FDA clearance marks another milestone for Asensus Surgical and represents an additional indication expansion in the U.S. market,” said Anthony Fernando, Asensus Surgical President and CEO. “The Senhance System's precision and advanced digital capabilities make it uniquely suited for urological procedures, offering surgeons the benefit of digital tools and smaller instrumentation. We are excited to bring this technology to urologists and their patients across the United States.”
Healthcare providers perform upwards of 185,000 urological surgical procedures each year in the United States.* While the Senhance System has been successfully utilized for Urology procedures outside the U.S. for several years, this expanded indication will open the door to this important specialty to leverage the advantages of the Senhance System in the U.S. patient population.
Chester Koh MD, MBA, FACS, FAAP, a pediatric urologist and director of the Pediatric Robotic Surgery Program and Professor at Baylor College of Medicine in Houston, Texas noted that, “With a full suite of 3mm and 5mm instruments and digital integrations, the Senhance Surgical System combines the benefits of robotics and minimally invasive surgery. This is an exciting step in the evolution of Urological surgery for both adult and pediatric patients.”
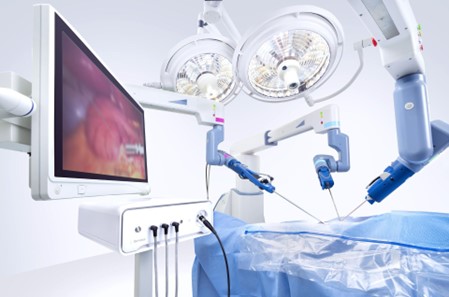
The Senhance Surgical System is designed to increase surgeon control and reduce variability through Augmented Intelligence and deep learning capabilities.
About Asensus Surgical, Inc.
Asensus Surgical is revolutionizing surgery with the first intra-operative Augmented Intelligence technology approved for use in operating rooms around the world. Recognized as an award-winning leader in digital technology, Asensus is committed to making surgery more accessible and predictable while delivering consistently superior outcomes. The Company’s novel approach to digitizing laparoscopy has led to system placements globally. Led by engineers, medical professionals, and industry luminaries, Asensus is powered by human ingenuity and driven by collaboration. To learn more about the Senhance® Surgical System and the new LUNA™ System in development, visit www.asensus.com.
Forward-Looking Statements
This press release includes statements relating to Asensus, the Senhance Surgical System and the FDA’s clearance for an expanded indication to treat adult and pediatric Urology patients with the Senhance Surgical System. These statements and other statements regarding our future plans and goals constitute "forward looking statements" within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, and are intended to qualify for the safe harbor from liability established by the Private Securities Litigation Reform Act of 1995. Such statements are subject to risks and uncertainties that are often difficult to predict, are beyond our control and which may cause results to differ materially from expectations and include whether the FDA clearance will represent another expansion in our market, and whether the Senhance Surgical System’s precision and advanced digital capabilities make it uniquely suited for urological procedures, offering surgeons the benefit of digital tools and smaller instrumentation. For a discussion of the risks and uncertainties associated with the Company’s business, please review our filings with the Securities and Exchange Commission (SEC), including our Annual Report on Form 10-K for the year ended December 31, 2023, filed with the SEC on March 21, 2024 and our other filings we make with the SEC. You are cautioned not to place undue reliance on these forward looking statements, which are based on our expectations as of the date of this press release and speak only as of the origination date of this press release. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise.
ASENSUS SURGICAL CONTACT:
INVESTORS
Mark Klausner or Mike Vallie
ICR Westwicke
invest@asensus.com
443-213-0499
MEDIA
Dan Ventresca
Matter Communications
AsensusPR@matternow.com
617-874-5488
*Urology Market data forecasted for 2023 from DRG Clarivate Laparoscopic Market Insights - December 2021
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/de156f18-f140-4fc1-86fd-ee125390d46e








