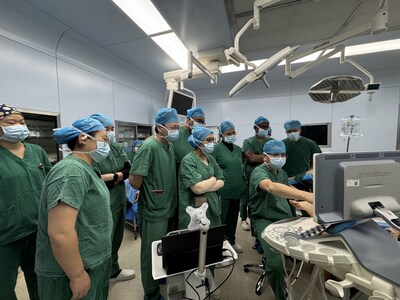
Baird Medical Successfully Concludes Gynecology Microwave Ablation Masterclass
Rhea-AI Summary
Baird Medical (NASDAQ: BDMD) concluded a Gynecological Microwave Ablation Masterclass in Shanghai on January 28, 2026, led by Dr. Xiaoming Gong. The program combined didactic lectures and live case observations to train physicians across Asia in the company's proprietary microwave ablation technology.
The event reinforces Baird Medical's focus on physician education, expansion of minimally invasive women's health treatments, and broader commercial presence, noting FDA 510(k) certification and use in over 30 U.S. hospitals and in more than 20 countries.
Positive
- None.
Negative
- None.
Key Figures
Market Reality Check
Peers on Argus
BDMD was down 0.97% while peers were mixed: APYX up 7.99%, ICAD up 3.48%, ICCM down 3.05%, NSPR down 0.6%, XTNT down 3.34%. With no peers in the momentum scanner and mixed directions, trading appeared stock-specific rather than a coordinated Medical Devices move.
Historical Context
| Date | Event | Sentiment | Move | Catalyst |
|---|---|---|---|---|
| Jan 26 | Strategic partnership | Positive | +0.0% | Partnership with Stonewood Key Capital to accelerate global MWA commercialization. |
| Jan 20 | Conference engagement | Positive | -0.9% | JP Morgan Healthcare Conference meetings on U.S. expansion and MWA adoption. |
| Jan 07 | Regulatory approvals | Positive | +3.9% | Product registrations in Pakistan and Vietnam enabling further MWA market entry. |
| Dec 17 | U.S. manufacturing | Positive | +2.2% | Establishment of U.S. manufacturing base with MPS Medical for global supply. |
| Dec 08 | Clinical training | Positive | -4.9% | Advanced MWA training for an Arizona surgeon with live procedures and planning. |
Recent operational and expansion news has produced mixed reactions, with both aligned and divergent price responses to generally positive developments.
Over the past two months, BDMD has focused on global expansion and clinical adoption of its Microwave Ablation (MWA) platform. On Dec 8, 2025, it highlighted advanced MWA training in Arizona, followed by establishing a U.S. manufacturing base on Dec 17, 2025. Regulatory approvals in Pakistan and Vietnam were announced on Jan 7, 2026, and strategic investor engagement at the 2026 J.P. Morgan Healthcare Conference came on Jan 20, 2026. A partnership with Stonewood Key Capital on Jan 26, 2026 advanced its global commercialization roadmap. Today’s Shanghai gynecology masterclass fits this ongoing push to deepen physician education and broaden minimally invasive adoption.
Market Pulse Summary
This announcement highlights BDMD’s focus on physician education and expanding minimally invasive microwave ablation into gynecological indications, complementing earlier regulatory wins and manufacturing expansion. The Shanghai masterclass reinforces a strategy centered on training and clinical adoption, adding to prior initiatives in the U.S. and new international markets. Investors may watch how such programs translate into broader procedure volumes and geographic uptake, especially given the stock’s pre-news level of $1.02 versus a $2.82 200-day moving average and $8.2888 52-week high.
Key Terms
microwave ablation medical
minimally invasive medical
AI-generated analysis. Not financial advice.
The event featured a combination of expert-led didactic lectures and live case observations, providing participating physicians from across
This initiative highlights Baird Medical's enduring commitment to physician education and the expansion of minimally invasive treatment options for women's health. Through programs like the Shanghai Masterclass, the Company continues to empower medical professionals with the skills necessary to deliver superior patient outcomes using state-of-the-art microwave ablation therapy.
About Baird Medical
Baird Medical is a forward-thinking medical device company specializing in minimally invasive diagnostics and treatment. It is dedicated to the research and development of surgical robotic systems and innovative minimally invasive surgical instruments. Our mission is to enhance patient outcomes through precision technology and advanced diagnostic solutions. The company will foster strategic collaborations with leading academic institutions. Our vision extends beyond surgical assistance, aiming to develop intelligent systems that proactively guide diagnostic decisions and preventive healthcare strategies. As an FDA 510(k)-certified medical device company, Baird Medical's solutions have been used in over 30 prestigious hospitals and clinics across
Forward-Looking Statements
This press release includes certain statements that are not historical facts but are forward-looking statements for purposes of the safe harbor provisions under the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements generally relate to future events or Baird Medical's future financial or operating performance. In some cases, you can identify forward-looking statements by terminology such as "may", "could", "should", "expect", "intend", "might", "will", "estimate", "anticipate", "believe", "budget", "forecast", "intend", "plan", "potential", "predict", or "continue", or the negatives of these terms or variations of them or similar terminology. Forward-looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward-looking statements. These forward-looking statements are based upon estimates and assumptions that, while considered reasonable by Baird Medical and its management, are inherently uncertain. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. You should not place undue reliance on forward-looking statements in this press release, which speak only as of the date they are made and are qualified in their entirety by reference to the cautionary statements herein. Baird Medical does not undertake any duty to update these forward-looking statements.
Actual results may vary materially from those expressed or implied by forward-looking statements based on a number of factors, including, without limitation: (1) the risk that Baird Medical may not be successful in expanding its business in China or the
The foregoing list of factors is not exclusive. Additional information concerning certain of these, and other risk factors is contained in ExcelFin's most recent filings with the SEC and in the Registration Statement described above filed by Baird Medical in connection with its business combination with ExcelFin. All subsequent written and oral forward-looking statements concerning Baird Medical, the business combination described herein or other matters attributable to Baird Medical or any person acting on its behalf are expressly qualified in their entirety by the cautionary statements above. Readers are cautioned not to place undue reliance upon any forward-looking statements, which speak only as of the date made. Baird Medical expressly disclaims any obligations or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in their expectations with respect thereto or any change in events, conditions or circumstances on which any statement is based.
Contact:
Eric Huang, PR Liaison
Baird Medical Investment Holdings Ltd.
Phone: +1 (888) 508-6228
Email: ir@bairdmed.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/baird-medical-successfully-concludes-gynecology-microwave-ablation-masterclass-302669438.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/baird-medical-successfully-concludes-gynecology-microwave-ablation-masterclass-302669438.html
SOURCE BDMD