Biodexa Announces Exclusive License of Otsuka’s OPB-171775, a potent Phase 1 ready Molecular Glue for GIST
Rhea-AI Summary
Biodexa (Nasdaq: BDRX) closed an exclusive license with Otsuka for OPB-171775, a Phase 1–ready molecular glue intended for treatment of gastrointestinal stromal tumours (GIST). Biodexa will develop the asset as MTX240, citing activity in TKI‑resistant PDX models and potential use in additional indications. The program aligns with Biodexa's GI/oncology pipeline alongside its Phase 3 eRapa program in Familial Adenomatous Polyposis.
Positive
- Exclusive license secured from Otsuka for OPB-171775
- OPB-171775 is Phase 1–ready for clinical development
- Preclinical activity observed in TKI‑resistant PDX models
- Program integrates with Biodexa's GI/oncology pipeline and Phase 3 eRapa
Negative
- No human clinical data yet; first-in-human studies still required
News Market Reaction
On the day this news was published, BDRX declined 11.04%, reflecting a significant negative market reaction. Argus tracked a peak move of +26.0% during that session. Argus tracked a trough of -37.6% from its starting point during tracking. Our momentum scanner triggered 21 alerts that day, indicating elevated trading interest and price volatility. This price movement removed approximately $125K from the company's valuation, bringing the market cap to $1M at that time. Trading volume was exceptionally heavy at 11.8x the daily average, suggesting significant selling pressure.
Data tracked by StockTitan Argus on the day of publication.
Key Figures
Market Reality Check
Peers on Argus
BDRX was down 4.12% while 2 momentum-screened biotech peers moved up (median gain about 6.3%). This opposite direction suggests stock-specific dynamics rather than a pure sector rotation.
Previous Clinical trial Reports
| Date | Event | Sentiment | Move | Catalyst |
|---|---|---|---|---|
| Dec 01 | Phase 3 enrolment update | Positive | +2.2% | First European patients enrolled in pivotal Phase 3 Serenta eRapa trial for FAP. |
| Nov 24 | Trial site activation | Positive | -1.4% | First European site activated for registrational Phase 3 Serenta FAP trial. |
| Nov 03 | CTA approval Europe | Positive | -2.4% | EMA CTA approval for Phase 3 Serenta FAP trial, outlining <b>$7B</b> addressable market. |
| Sep 08 | Phase 3 initiation | Positive | -5.8% | Move into Phase 3 eRapa FAP trial with added grant funding and strong Phase 2 data. |
| Aug 18 | First Phase 3 patients | Positive | +2.8% | First patients enrolled in pivotal Phase 3 Serenta trial targeting FAP. |
Clinical trial news for Biodexa has produced mixed reactions, with more instances of negative than positive 24h moves despite generally constructive clinical updates.
Over the last six months, Biodexa has focused on advancing eRapa for FAP through pivotal Phase 3 Serenta milestones, including first patient enrolment on Aug 18, 2025, moving into Phase 3 with added grant funding on Sep 8, 2025, and European CTA approval on Nov 3, 2025. Subsequent European site activation and first European patients on Nov 24 and Dec 1, 2025 further built this program. Today’s OPB‑171775 (MTX240) license expands the GI/oncology pipeline alongside this established Phase 3 FAP effort.
Historical Comparison
Past 12 months saw 5 clinical/FAP trial updates with an average -0.91% move, showing that even constructive clinical milestones have not consistently driven sustained gains.
Historical clinical news tracks steady Phase 3 Serenta progress for eRapa in FAP, while the OPB‑171775 (MTX240) license adds an earlier-stage GI/oncology asset alongside this late-stage program.
Market Pulse Summary
The stock dropped -11.0% in the session following this news. A negative reaction despite constructive pipeline expansion would fit Biodexa’s history, where several positive Phase 3 Serenta updates saw 24‑hour declines despite advancing FAP development. Prior equity offerings and large outstanding share counts have also weighed on sentiment. Investors have often treated good clinical news with caution, suggesting sensitivity to financing risk and execution rather than the news flow alone.
Key Terms
molecular glue medical
gastrointestinal stromal tumours medical
TKI medical
PDX models medical
AI-generated analysis. Not financial advice.
February 4, 2026
Biodexa Announces Exclusive License of Otsuka’s OPB-171775,
a potent Phase 1 ready Molecular Glue for GIST
Novel Mechanism of Action Shown to be Effective in TKI Resistant PDX Models
Cardiff, UK – February 4, 2026 – Biodexa Pharmaceuticals PLC (Nasdaq: BDRX), a clinical stage biopharmaceutical company developing a pipeline of innovative products for the treatment of rare diseases with unmet medical needs, today announced the closing of an exclusive license with Otsuka Pharmaceutical Co., Ltd (Otsuka) for OPB-171775. a novel molecular glue intended to be developed for the treatment of gastrointestinal stromal tumours (GIST). The compound also has the potential to be useful in additional indications. In Biodexa’s pipeline, OPB-171775 is to be coded MTX240.
Stephen Stamp, Chief Executive Officer of Biodexa commented "MTX240 represents a fantastic opportunity for Biodexa. Its unique molecular glue mechanism of action separates MTX240 from current standard of care with the potential to benefit a broad spectrum of GIST patients, including those with TKI-resistant disease. MTX240 strategically aligns with our emerging GI/oncology pipeline which includes our ongoing Phase 3 development of eRapa in Familial Adenomatous Polyposis."
About MTX240, its Unique Mechanism of Action and Clinical Rationale
Molecular glue technology represents a novel approach that induces targeted protein interactions, offering a distinct mechanism of action to conventional kinase inhibitors for rare oncology indications.
GIST is driven by activating mutations in the KIT receptor tyrosine kinase. While tyrosine kinase inhibitors (TKIs) such as imatinib, sunitinib, and regorafenib are reported to have significantly improved outcomes for GIST patients, resistance almost always develops through secondary KIT mutations or activation of alternative signalling pathways. This represents a substantial clinical challenge with limited therapeutic options for patients once they have cycled through the available TKIs.
MTX240 acts as a molecular glue, bringing two intracellular proteins, PDE3a and SLFN12, specifically co-expressed by GIST cancer cells, into close proximity to form a stable complex. This interaction stabilizes SLFN12, enabling it to drive RNase-mediated apoptosis in GIST cells through a mechanism independent of KIT signalling. By triggering cell death through this alternative pathway, MTX240 is designed to overcome the resistance mechanisms that render TKI-resistant GISTs refractory to conventional kinase inhibitors. This novel mechanism may provide clinical benefit for a significant proportion of GIST patients, not only those who have developed resistance to TKIs.
In patient derived xenograft (PDX) mice, MTX240 has shown dose-dependent anti-tumor efficacy in imatinib and sunitinib resistant models irrespective of KIT mutation status as demonstrated by E.O. Takaki et al1. in the following charts:
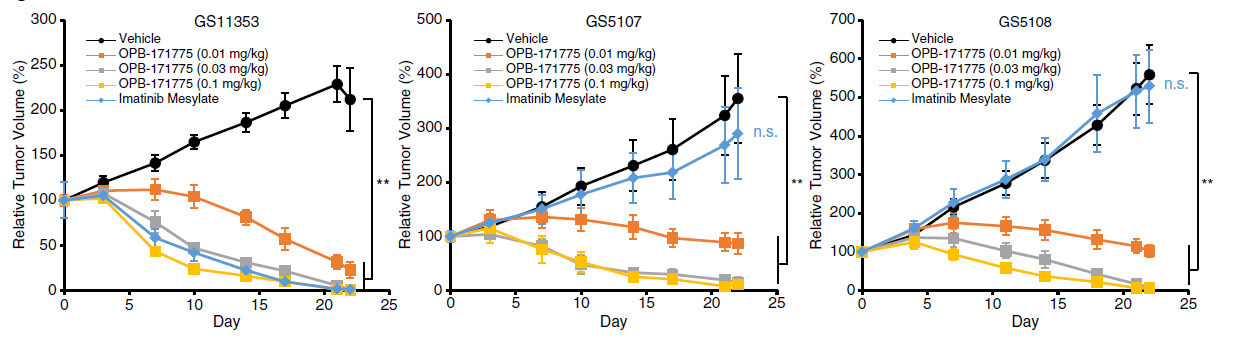
MTX240, referenced as OPB-171775 in the charts, and vehicle were administered once daily for 21 days. Imatinib was administered twice daily for 21 days. The values represent mean tumor volumes +/- standard error of the mean GS5107 (n=3), GS5108 (n=5), GS11353 (n=5).
PDX models use actual tumor tissue harvested from patients, preserving the genetic complexity and treatment resistance patterns of real human cancers. This makes PDX studies far more predictive of human clinical outcomes because they bridge the gap between laboratory discovery and clinical trials5.
Addressing Unmet Medical Need in GIST
GIST is a rare gastrointestinal malignancy affecting approximately 3,000-4,000 patients annually in the United States2, with a significant unmet medical need for patients who develop TKI resistance. Approximately 10
The global GIST market is valued at approximately USD 1.3 billion and is expected to grow at 6
GIST qualifies for orphan drug designation in major regulatory jurisdictions, offering potential regulatory advantages and incentives to support drug development.
Licensing and Collaboration Agreement
Under the terms of the license agreement Biodexa has the exclusive rights to develop and commercialize MTX240 globally with the exception of Japan where Otsuka retains its rights. The agreement includes an upfront fee and additional development and regulatory milestones. In addition, tiered royalties in the mid-single digit range are payable on net sales of MTX240.
MTX240 benefits from composition of matter patents in the US, Europe, Japan and various other countries extending through 2037 without any patent term extension.
- Takaki EO, Kiyono K, Obuchi Y, et al. A PDE3A-SLFN12 Molecular Glue Exhibits Significant Antitumor Activity in TKI-Resistant Gastrointestinal Stromal Tumors. Clinical Cancer Research. 2024;30(16):3603–3621.
- Datar R, et al. Inpatient burden of gastrointestinal stromal tumors in the United States. American Journal of Clinical Oncology. 2012.
- Kee D, et al. Current and emerging strategies for the management of gastrointestinal stromal tumors. Nature Clinical Practice Oncology. 2012;9(1):24-33.
- Future Market Insights. Gastrointestinal Stromal Tumor (GIST) Therapeutics Market. September 2, 2025
- Floc'h N, et al. Optimizing the design of population-based patient-derived xenograft studies to predict drug efficacy in patient populations. Nature Communications. 2018;9:4608
For more information, please contact:
Biodexa Pharmaceuticals PLC |
| Stephen Stamp, CEO Steve Ellul, CBO |
| Tel: +44 (0)29 20480 180 |
| www.biodexapharma.com |
About Biodexa Pharmaceuticals PLC
Biodexa Pharmaceuticals PLC (listed on NASDAQ: BDRX) is a clinical stage biopharmaceutical company developing a pipeline of innovative products for the treatment of diseases with unmet medical needs. The Company’s lead development programs include eRapa, under development for Familial Adenomatous Polyposis and Non-Muscle Invasive Bladder Cancer and tolimidone, under development for the treatment of type 1 diabetes.
eRapa is a proprietary oral capsule formulation of rapamycin, also known as sirolimus. Rapamycin is an mTOR (mammalian Target Of Rapamycin) inhibitor. mTOR has been shown to have a significant role in the signalling pathway that regulates cellular metabolism, growth and proliferation and is activated during tumorigenesis.
Tolimidone is an orally delivered, potent and selective inhibitor of Lyn kinase. Lyn is a member of the Src family of protein tyrosine kinases, which is mainly expressed in hematopoietic cells, in neural tissues, liver, and adipose tissue. Tolimidone demonstrates glycaemic control via insulin sensitization in animal models of diabetes and has the potential to become a first in class blood glucose modulating agent.
Biodexa’s headquarters and R&D facility is in Cardiff, UK. For more information visit www.biodexapharma.com.
Forward-Looking Statements
Certain statements in this announcement may constitute “forward-looking statements” within the meaning of legislation in the United Kingdom and/or United States. Such statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and are based on management’s belief or interpretation. All statements contained in this announcement that do not relate to matters of historical fact should be considered forward-looking statements. In certain cases, forward-looking statements can be identified by the use of words such as “plans”, “expects” or “does not anticipate”, or “believes”, or variations of such words and phrases or statements that certain actions, events or results “may”, “could”, “would”, “might” or “will be taken”, “occur” or “be achieved.” Forward-looking statements and information are subject to various known and unknown risks and uncertainties, many of which are beyond the ability of the Company to control or predict, that may cause their actual results, performance or achievements to be materially different from those expressed or implied thereby, and are developed based on assumptions about such risks, uncertainties and other factors set out herein.
Reference should be made to those documents that Biodexa shall file from time to time or announcements that may be made by Biodexa in accordance with the rules and regulations promulgated by the SEC, which contain and identify other important factors that could cause actual results to differ materially from those contained in any projections or forward-looking statements. These forward-looking statements speak only as of the date of this announcement. All subsequent written and oral forward-looking statements by or concerning Biodexa are expressly qualified in their entirety by the cautionary statements above. Except as may be required under relevant laws in the United States, Biodexa does not undertake any obligation to publicly update or revise any forward-looking statements because of new information, future events or events otherwise arising.








