BioAge Labs completes IND-enabling studies for BGE-102, a potent, orally available, brain-penetrant NLRP3 inhibitor, and advances candidate toward the clinic
Rhea-AI Summary
Positive
- Preclinical studies showed 15% weight reduction with BGE-102 monotherapy, comparable to semaglutide
- Combination with semaglutide achieved over 20% weight reduction, showing additive effects
- High potency with 2 nM IC90 in human microglia and favorable brain penetration ratio of ~1
- Strong safety profile with 35-75x safety margin for predicted human dose
- Low predicted daily dose (<50 mg) with convenient once-daily oral administration
Negative
- Clinical trials haven't started yet - still in preclinical stage
- Phase 1b proof-of-concept study won't begin until second half of 2026
- Efficacy in humans remains unproven despite promising preclinical results
News Market Reaction
On the day this news was published, BIOA declined NaN%, reflecting a moderate negative market reaction.
Data tracked by StockTitan Argus on the day of publication.
IND submission planned for mid-2025 with Phase 1 SAD data anticipated by year end
BGE-102 induced weight loss in preclinical obesity models, both as monotherapy and in combination with GLP-1 receptor agonists
Internally discovered compound has a novel binding site and potential best-in-class profile supporting once daily dosing
EMERYVILLE, Calif., May 29, 2025 (GLOBE NEWSWIRE) -- BioAge Labs, Inc. ("BioAge", "the Company"), a clinical-stage biotechnology company developing therapeutic product candidates for metabolic diseases by targeting the biology of human aging, today announced completion of IND-enabling studies for BGE-102 and updated clinical development milestones for the compound. BGE-102 is a structurally novel, orally available small-molecule NLRP3 inhibitor with high potency and brain penetration. BioAge is initially developing BGE-102 for the treatment of obesity.
Chronic activation of the NLRP3 inflammasome due to aging, cellular stress, or nutrient excess can lead to inflammation that drives various diseases. BioAge identified NLRP3 as a therapeutic target based on analysis of human aging cohorts by the Company’s platform, which revealed that reduced NLRP3 activity is associated with greater longevity. In obesity, NLRP3 activation contributes to both neuroinflammation, which disrupts appetite regulation, and systemic inflammation, which is elevated in patients with obesity and is a key risk factor for cardiovascular disease.
BGE-102 potently induces weight loss as monotherapy and in combination with incretins in obese mice
In preclinical models of obesity, BGE-102 monotherapy produced dose-dependent weight loss similar in magnitude to semaglutide, with optimal doses achieving approximately
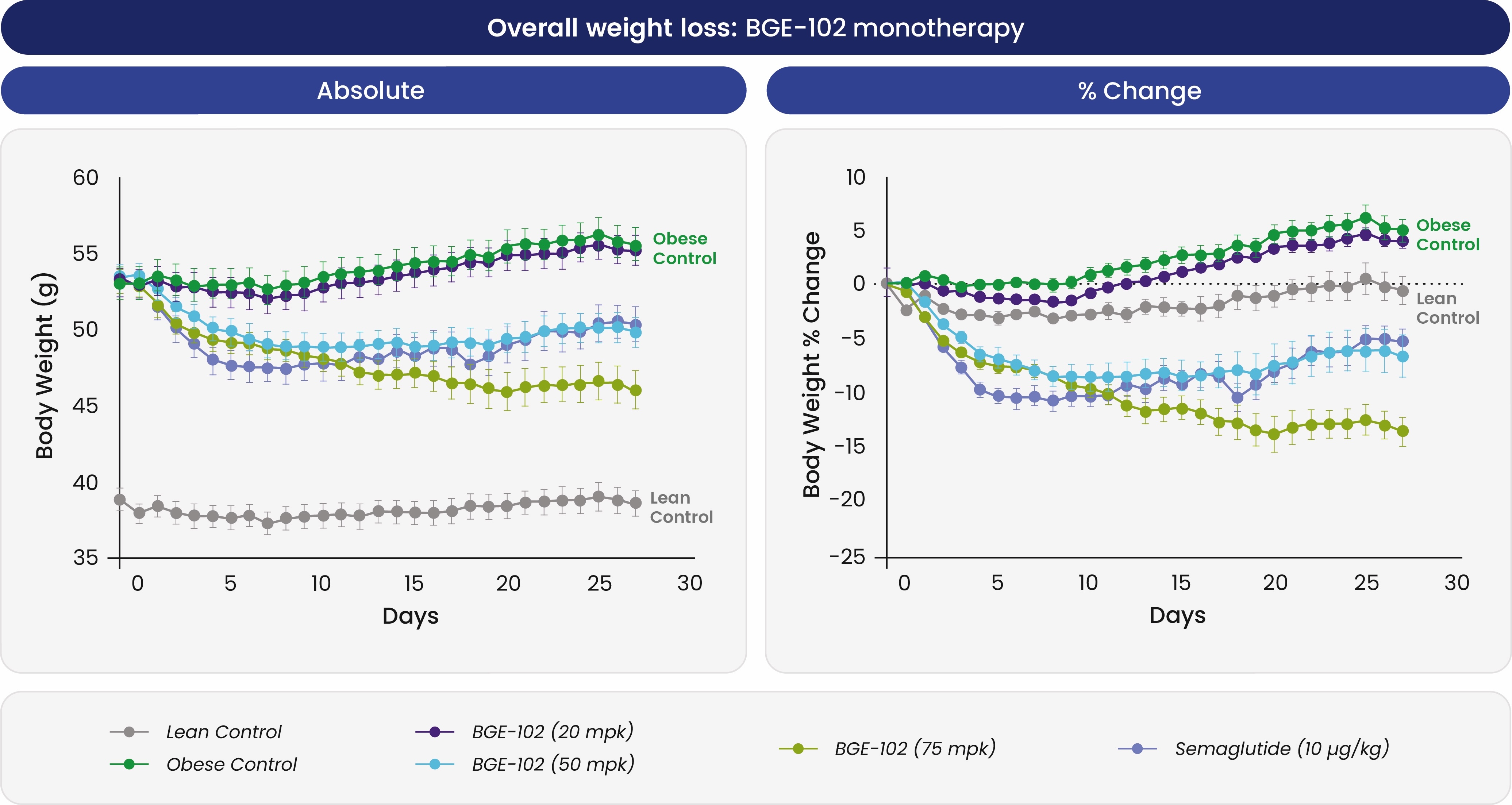
Figure 1. BGE-102 resulted in significant, dose-dependent increases in overall weight loss compared to placebo in a diet-induced obesity mouse model. Group size: n=8–10 per group. BGE-102 75 mpk vs. obese control, Day 27: p<0.0001. Note: BGE-102 is 150–250x more potent in human microglia, the target cell in the brain, compared to mouse microglia, consistent with <50 mg predicted daily dose.
When combined with semaglutide, BGE-102 produced additive weight loss effects, with the combination achieving over
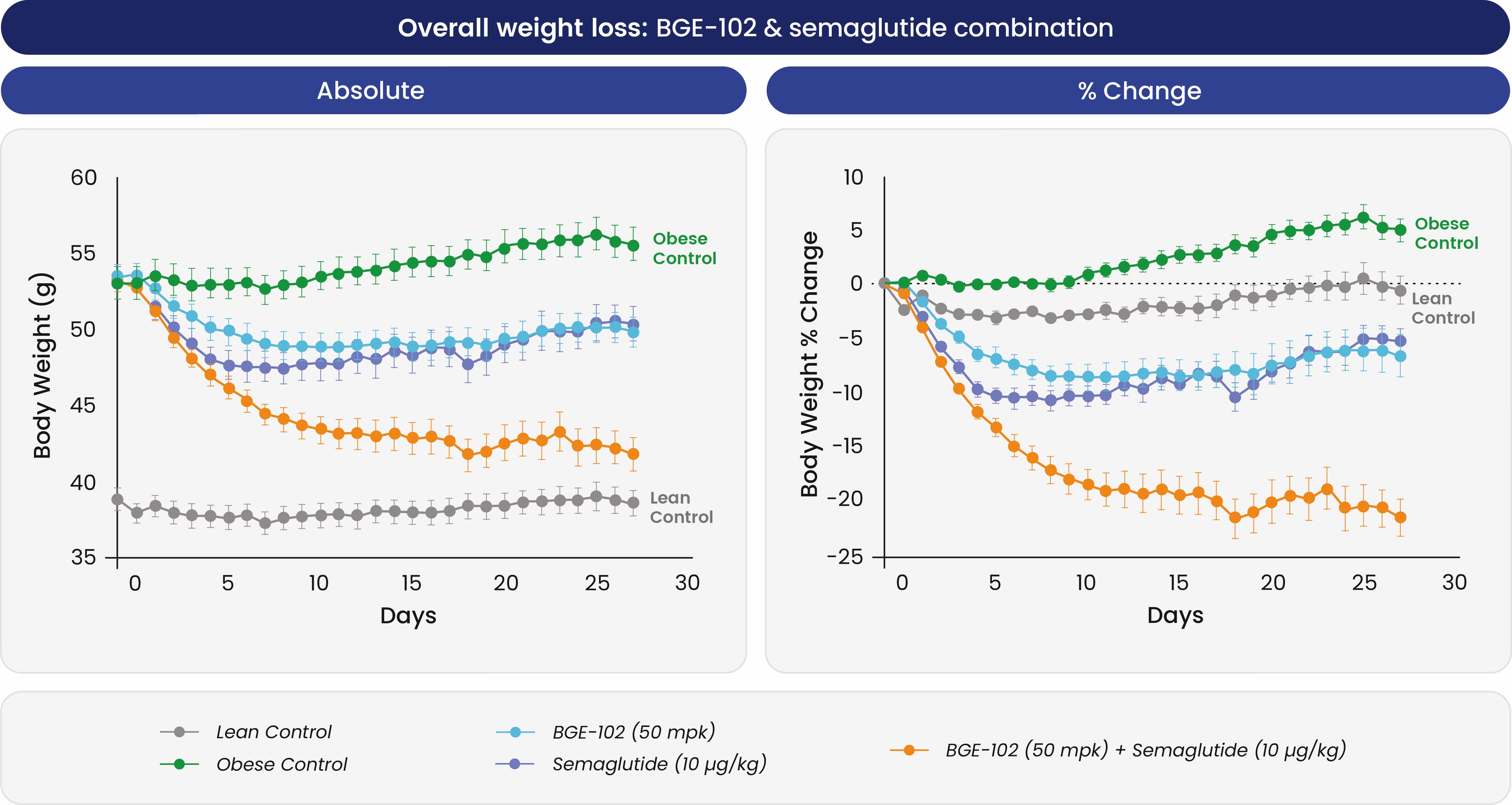
Figure 2. BGE-102 resulted in significant additive weight loss when combined with GLP-1R agonist semaglutide. Group size: n=8–10 per group. BGE-102 50 mpk + semaglutide vs. obese control, Day 27: p<0.0001. Note: BGE-102 is 150–250x more potent in human microglia, the target cell in the brain, compared to mouse microglia, consistent with <50 mg predicted daily dose.
“By inhibiting the NLRP3 inflammasome, BGE-102 targets a core pathway that links metabolism, inflammation, and aging,” said Kristen Fortney, Ph.D., CEO and co-founder of BioAge. “Its potential potency, brain penetration, and pharmacokinetics which suggest the possibility for once-daily dosing position BGE-102 as a convenient oral therapy for obesity—either alone or alongside GLP-1 receptor agonists—and may unlock additional opportunities in diseases driven by NLRP3-mediated inflammation.”
Potential best-in-class molecular profile with high potency, brain penetration, and attractive safety margin
BGE-102 features a novel structure with a unique binding site that differentiates it from other NLRP3 inhibitors in development. The compound is highly brain penetrant with a brain-to-plasma ratio of approximately 1, and highly potent with a 2 nM IC90 in human microglia, the target cells in the brain. The compound's potency and pharmacokinetic properties are consistent with the potential to achieve greater than
BGE-102 has demonstrated a favorable preclinical safety profile with no adverse findings in in vitro safety assessments, safety pharmacology, and behavioral studies. Toxicology studies have shown a 35–75x safety margin for the predicted human dose, with no adverse effects observed in 28-day GLP toxicology studies.
Upcoming clinical development milestones
BioAge plans to submit an Investigational New Drug (IND) application for BGE-102 in mid-2025. Following IND clearance, the Company expects to initiate a Phase 1 single ascending dose (SAD) and multiple ascending dose (MAD) clinical trial, with initial SAD data anticipated by the end of 2025.
Following successful Phase 1 results, BioAge plans to initiate a Phase 1b proof-of-concept study in obesity in the second half of 2026.
About BioAge Labs, Inc.
BioAge is a clinical-stage biopharmaceutical company developing therapeutic product candidates for metabolic diseases by targeting the biology of human aging. The Company's lead product candidate, BGE-102, is a potent, orally available, brain-penetrant small-molecule NLRP3 inhibitor being developed for diseases driven by inflammation, including obesity. BGE-102 has demonstrated significant weight loss in preclinical models both as monotherapy and in combination with GLP-1 receptor agonists. IND submission and initiation of a Phase 1 SAD/MAD trial are planned for mid-2025, with initial SAD data anticipated by end of year. The Company’s pipeline also includes novel APJ agonists. BioAge’s additional preclinical programs, which leverage insights from the Company’s proprietary discovery platform built on human longevity data, address key pathways involved in metabolic aging.
Forward-looking statements
This press release contains “forward-looking statements” within the meaning of, and made pursuant to the safe harbor provisions of, the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including, but not limited to, statements regarding our plans to develop and commercialize our product candidates, including BGE-102, the timing and results of our planned clinical trials, risks associated with clinical trials, including our ability to adequately manage clinical activities, the timing of our IND filing for BGE-102 and our ability to obtain and maintain regulatory approvals, the clinical utility of our product candidates, and general economic, industry and market conditions. These forward-looking statements may be accompanied by such words as “aim,” “anticipate,” “believe,” “could,” “estimate,” “expect,” “forecast,” “goal,” “intend,” “may,” “might,” “plan,” “potential,” “possible,” “will,” “would,” and other words and terms of similar meaning. These statements involve risks and uncertainties that could cause actual results to differ materially from those reflected in such statements, including: our ability to develop, obtain regulatory approval for and commercialize our product candidates; the timing and results of preclinical studies and clinical trials; the risk that positive results in a preclinical study or clinical trial may not be replicated in subsequent trials or success in early stage clinical trials may not be predictive of results in later stage clinical trials; risks associated with clinical trials, including our ability to adequately manage clinical activities, unexpected concerns that may arise from additional data or analysis obtained during clinical trials, regulatory authorities may require additional information or further studies, or may fail to approve or may delay approval of our drug candidates; the occurrence of adverse safety events; failure to protect and enforce our intellectual property, and other proprietary rights; failure to successfully execute or realize the anticipated benefits of our strategic and growth initiatives; risks relating to technology failures or breaches; our dependence on collaborators and other third parties for the development of product candidates and other aspects of our business, which are outside of our full control; risks associated with current and potential delays, work stoppages, or supply chain disruptions, including due to the imposition of tariffs and other trade barriers; risks associated with current and potential future healthcare reforms; risks relating to attracting and retaining key personnel; changes in or failure to comply with legal and regulatory requirements, including shifting priorities within the U.S. Food and Drug Administration; risks relating to access to capital and credit markets; and the other risks and uncertainties that are detailed under the heading “Risk Factors” included in BioAge’s Quarterly Report on Form 10-Q filed with the U.S. Securities and Exchange Commission (SEC) on May 6, 2025, and BioAge’s other filings with the SEC filed from time to time. BioAge undertakes no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise.
Contacts
PR: Chris Patil, media@bioagelabs.com
IR: Dov Goldstein, ir@bioagelabs.com
Partnering: partnering@bioagelabs.com
Web: https://bioagelabs.com
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/48a633db-fdf1-45da-b08e-f9f933cf64e4
https://www.globenewswire.com/NewsRoom/AttachmentNg/63613b30-dc29-42d5-8438-b60bed365342








