New Hope for People Living with a Disease Once Deemed Untreatable: Belite Bio Announces Positive Topline Results from the Pivotal Global, Phase 3 DRAGON Trial of Tinlarebant in Adolescents with Stargardt Disease
Rhea-AI Summary
Belite Bio (NASDAQ: BLTE) reported positive topline results from the global Phase 3 DRAGON trial of Tinlarebant in adolescent Stargardt disease (Dec 1, 2025). The trial (n=104) met its primary endpoint, showing a 35.7% reduction in retinal lesion growth versus placebo (p=0.0033) with supportive post‑hoc analyses (p<0.0001). Key secondary endpoints and fellow‑eye analyses were also statistically significant. Tinlarebant (5 mg daily) reduced RBP4 ~80% and was generally well tolerated. The company plans to file an NDA in 1H 2026.
Positive
- Primary endpoint met: 35.7% lesion growth reduction (p=0.0033)
- Fellow eye benefit: 33.6% lesion growth reduction (p=0.041)
- Key secondary endpoint DAF reduced 33.7% in study eye (p=0.027)
- Pharmacology: RBP4 reduced ~80% on 5 mg daily dose
- Regulatory: plans to file NDA in 1H 2026; multiple orphan/pediatric designations
Negative
- Minimal change in visual acuity over 24 months in both groups
- Four treatment‑related discontinuations reported
- Common ocular adverse events: xanthopsia and delayed dark adaptation
Insights
Positive pivotal Phase 3 topline — Tinlarebant met its primary endpoint and supports an NDA submission in
Tinlarebant produced a statistically significant reduction in lesion growth versus placebo (primary endpoint p = 0.0033) with an effect size of ~
The regulatory pathway now hinges on the full dataset and FDA interactions; the company intends to file an NDA in
Watch for the full dataset release, regulatory guidance outcomes, and data presented at upcoming medical meetings over the next
- Tinlarebant is the first therapeutic candidate to demonstrate clinical efficacy in a global Phase 3 trial for Stargardt disease, achieving a statistically significant p-value of 0.0033
- Tinlarebant met the primary efficacy endpoint, demonstrating clinical benefit by significantly reducing the lesion growth rate by
36% compared to placebo, as measured by retinal imaging - Tinlarebant was well tolerated throughout the trial
- Stargardt disease impacts more than 50,000 patients in the U.S.
- Belite Bio plans to file an NDA with the US FDA in 1H 2026
- Company will host a conference call and webcast today at 8:00 a.m. ET
SAN DIEGO, Dec. 01, 2025 (GLOBE NEWSWIRE) -- Belite Bio, Inc (NASDAQ: BLTE) (“Belite Bio” or the “Company”) today announced topline results from the global Phase 3 “DRAGON” trial of Tinlarebant, marking the first successful pivotal trial in patients with Stargardt disease type 1 (STGD1). STGD1 is an eye disease that leads to progressive vision loss, usually beginning in childhood or young adulthood, and currently has no approved treatment worldwide.
The Phase 3 DRAGON trial enrolled 104 patients with STGD1 and met its primary efficacy endpoint, demonstrating a statistically significant and clinically meaningful
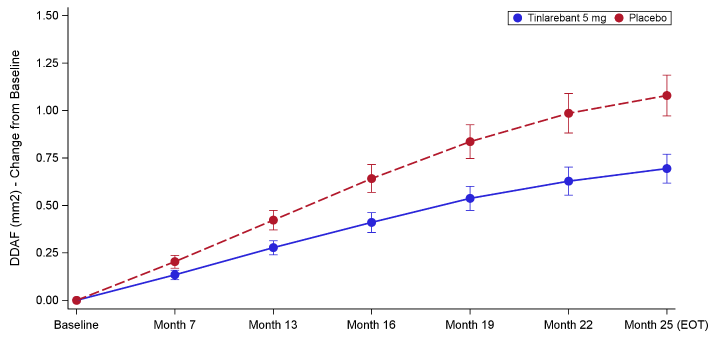
Figure: DDAF (mm2) - Least Squares Mean Change from Baseline by Visit - Study Eye
“The final results from the DRAGON trial mark a historic breakthrough in Stargardt disease, paving the way for the first potential treatment for this devastating condition and bringing new hope to patients and families who have long faced a disease once considered untreatable,” said Dr. Tom Lin, Chairman and CEO of Belite Bio. “Not only was Tinlarebant shown to be efficacious in slowing retinal degeneration, but this is also the first time that an oral treatment was able to demonstrate a clinically meaningful outcome in retinal degenerative disease. With this data, we are advancing our regulatory interactions globally and moving closer to delivering the first approved treatment for people living with Stargardt disease. We extend our sincere gratitude to the patients, families, and investigators whose dedication made this achievement possible.”
“The significant lesion growth reduction observed in the DRAGON study, along with the favorable safety profile, provide important validation of our therapeutic approach and the mechanism of Tinlarebant. These results underscore the team’s commitment to addressing the unmet need in Stargardt disease and the potential to meaningfully improve the quality of life for those affected,” said Dr. Nathan Mata, Chief Scientific Officer at Belite Bio.
As expected, the overall change in visual acuity was minimal over the period of 24 months in both study groups, consistent with natural history data. The safety profile remains consistent with what the Company previously reported, and Tinlarebant was well tolerated with only four treatment-related discontinuations. After the full analysis is complete, the Company plans to share additional data at upcoming medical meetings.
“Seeing well-controlled Phase 3 data that shows a marked slowing of lesion growth in Stargardt disease is deeply encouraging,” said Professor Michel Michaelides, M.D., FRCOphth, Consultant Ophthalmologist at Moorfields Eye Hospital, the leading investigator in the UK and a top enroller in the DRAGON trial. “Given the strength and consistency of these findings, we believe an approved treatment option is on the horizon for people living with this devastating condition.”
“It is remarkable to recognize that with the robust results of the DRAGON trial, we may soon have Tinlarebant as the first treatment ever for Stargardt disease,” noted Quan Dong Nguyen, MD, MSc, FAAO, FARVO, FASRS, Professor of Ophthalmology at the Byers Eye Institute at Stanford, and Professor of Medicine and Pediatrics at Stanford University School of Medicine. “It is only a matter of time before the observed reduction in lesion growth translates into measurable benefits in visual function. Previous studies have demonstrated that if left untreated, progressive lesion enlargement caused by Stargardt disease is expected to compromise visual acuity and visual field. Tinlarebant has demonstrated that it can significantly reduce lesion growth.”
“The DRAGON trial delivers the most compelling evidence to date that an oral therapy can alter the course of Stargardt disease,” said Dr. Hendrik Scholl, Chief Medical Officer of Belite Bio. “These results validate the scientific approach behind Tinlarebant’s development, demonstrating that reducing the accumulation of toxic byproducts in the retina can meaningfully slow disease progression. Tinlarebant produced a clear and statistically significant treatment effect on DDAF lesion growth rate, not only in the study eye, but both eyes. Furthermore, the effect was supported by a statistically significant benefit in the key secondary endpoint, again in both eyes. Safety and tolerability of Tinlarebant was very favorable in the DRAGON trial. Collectively, these data reinforce Tinlarebant’s potential to change the treatment landscape for Stargardt disease and set a new benchmark for future research in inherited retinal disorders.”
Regulatory Highlights
The Company plans to engage regulatory authorities to discuss potential next steps and to submit New Drug Applications for Tinlarebant in the first half of 2026. Tinlarebant has been granted Breakthrough Therapy, Fast Track, and Rare Pediatric Disease Designations in the U.S.; Orphan Drug Designation in the U.S., Europe, and Japan; and Pioneer Drug Designation in Japan for STGD1.
DRAGON Data Highlights
The DRAGON trial was a 24-month, randomized (2:1, active: placebo), double-masked, placebo-controlled, global, multi-center, pivotal Phase 3 trial in adolescent STGD1 patients.
Patient Demographics
- 104 patients (n=69 in tinlarebant arm and n=35 in placebo arm), ranging in age from 12-20 years, were enrolled in the DRAGON trial.
- All patients had been diagnosed with STGD1 with at least one mutation identified in the ABCA4 gene, an atrophic lesion size within three disc areas (7.62 mm2), and a best corrected visual acuity (BCVA) of 20/200 or better.
Positive Efficacy Results
- Tinlarebant achieved the primary efficacy endpoint demonstrating a statistically significant reduction in lesion growth rate of
35.7% versus placebo (p-value of 0.0033) as measured by retinal imaging, when applying an unstructured covariance matrix under the Mixed Model for Repeated Measures (MMRM). To account for the longitudinal nature of the collected data while maintaining model stability given the sample size in the DRAGON trial, a post-hoc analysis using an autoregressive covariance matrix under MMRM yielded a treatment effect size of35.4% with a p-value of <0.0001. - A statistically significant treatment effect was also observed in the fellow eye for the primary endpoint with
33.6% lesion growth reduction (p = 0.041). - In addition, Tinlarebant slowed decreased autofluorescence (DAF) lesion growth, the key secondary endpoint calculated as the sum of DDAF and questionably decreased autofluorescence (QDAF), in the study eye by
33.7% (p = 0.027) and in the fellow eye by32.7% (p = 0.017). - The 5 mg daily dose achieved a reduction in RBP4 levels by a mean of approximately
80% relative to baseline. - Retinal binding protein 4 (RPB4) levels returned to
84% of the baseline value at End of Study (one- to three-months following drug cessation). Recovery of RBP4 concentration correlated well with the decreased Tinlarebant exposure.
Strong Safety Profile Consistent with Past Trials
- Tinlarebant (5 mg orally, daily) was well tolerated in adolescent STGD1 patients.
- There were no drug or trial discontinuations due to non-ocular adverse events (AE). There were 4 drug discontinuations that were related to the treatment.
- Xanthopsia and delayed dark adaptation are the most common drug-related ocular AE. The majority of xanthopsia, delayed dark adaptation, and night vision impairment were mild, and most resolved during the trial.
- Headaches were the most commonly reported treatment-related non-ocular AE.
Webcast Information
Date: December 1, 2025
Time: 8:00 a.m. Eastern Time (5:00 a.m. Pacific Time)
Webcast Link: https://events.q4inc.com/attendee/851809284
Webcast Link Instructions
You can join the live webcast by visiting the link above or the “Presentations & Events” section of the Company’s Investor Relations website at https://investors.belitebio.com/presentations-events/events. A replay will be available following the event.
About Tinlarebant (a/k/a LBS-008)
Tinlarebant is a novel oral therapy that is intended to reduce the accumulation of vitamin A-based toxins (known as bisretinoids) that cause retinal disease in STGD1 and also contribute to disease progression in geographic atrophy (GA), or advanced dry age-related macular degeneration (AMD). Bisretinoids are by-products of the visual cycle, which is dependent on the supply of vitamin A (retinol) to the eye. Tinlarebant works by reducing and maintaining levels of serum retinol binding protein 4 (RBP4), the sole carrier protein for retinol transport from the liver to the eye. By modulating the amount of retinol entering the eye, Tinlarebant reduces the formation of bisretinoids. Tinlarebant has been granted Breakthrough Therapy Designation, Fast Track Designation and Rare Pediatric Disease Designation in the U.S., Orphan Drug Designation in the U.S. Europe, and Japan, and Sakigake Designation in Japan for the treatment of STGD1.
About Belite Bio
Belite Bio is a clinical-stage drug development company focused on advancing novel therapeutics targeting degenerative retinal diseases that have significant unmet medical need, such as Stargardt disease type 1 (STGD1) and Geographic Atrophy (GA) in advanced dry age-related macular degeneration (AMD), in addition to specific metabolic diseases. Belite’s lead candidate, Tinlarebant, an oral therapy intended to reduce the accumulation of toxins in the eye, has completed a Phase 3 trial (DRAGON) in adolescent STGD1 subjects and is currently being evaluated in a Phase 2/3 trial (DRAGON II) in adolescent STGD1 subjects and a Phase 3 trial (PHOENIX) in subjects with GA. For more information, follow us on X, Instagram, LinkedIn, and Facebook or visit us at www.belitebio.com.
Important Cautions Regarding Forward Looking Statements
This press release contains forward-looking statements about future expectations and plans, as well as other statements regarding matters that are not historical facts. These statements include but are not limited to statements regarding the potential implications of clinical data for patients, and Belite Bio’s advancement of, and anticipated preclinical activities, clinical development, regulatory milestones, timing of regulatory filings, and commercialization of its product candidates, the ability of Tinlarebant to treat STGD1 and GA, and any other statements containing the words “expect”, “hope” and similar expressions. Actual results may differ materially from those indicated in the forward-looking statements as a result of various important factors, including but not limited to Belite Bio’s ability to demonstrate the safety and efficacy of its drug candidates; the clinical results for its drug candidates, which may not support further development or regulatory approval; the timing to complete any ancillary clinical trials and/or to receive the interim/final data of such clinical trials; the timing to communicate with and submit trial data to regulatory authorities in various jurisdictions for drug approval; the content and timing of decisions made by the relevant regulatory authorities regarding regulatory approval of Belite Bio’s drug candidates; timing for Belite Bio to share additional data at upcoming medical meetings; the potential efficacy of Tinlarebant to set a new benchmark for future research in inherited retinal disorders, as well as those risks more fully discussed in the “Risk Factors” section in Belite Bio’s filings with the U.S. Securities and Exchange Commission. All forward-looking statements are based on information currently available to Belite Bio, and Belite Bio undertakes no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required by law.
Media and Investor Relations Contact:
Jennifer Wu /ir@belitebio.com
Sophie Hunt /belite@argotpartners.com
A photo accompanying this announcement is available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/2dd98cdb-8220-4814-aea4-0ea5400c2325








