Cognition Therapeutics Reports Topline Results Showing Oral Zervimesine (CT1812) Reduced Lesion Growth in Phase 2 Study in Geographic Atrophy
Rhea-AI Summary
Positive
- Zervimesine showed 28.6% reduction in GA lesion growth rate compared to placebo
- Treatment resulted in 28.2% smaller lesions at 18 months vs placebo
- Oral administration offers advantage over current injectable treatments
- Potential for use as monotherapy or in combination with existing medications
- Results validate drug's potential across multiple degenerative disease indications
Negative
- Study concluded with only 100 of planned 246 participants enrolled
- Complete safety data and visual outcomes not yet available
- Only 1/3 of participants completed the full 18-month treatment period
Insights
Cognition's oral zervimesine shows promising 28% reduction in GA lesion growth/size, potentially offering advantage over injectable treatments pending full data analysis.
Cognition Therapeutics has reported compelling Phase 2 data for zervimesine in geographic atrophy (GA), showing 28.6% slower lesion growth and 28.2% smaller lesion size at 18 months versus placebo. These numbers are particularly noteworthy when considering current FDA-approved GA treatments (complement inhibitors like Syfovre and Izervay) typically show lesion growth reductions in the 14-19% range in their pivotal studies.
The most disruptive aspect of zervimesine is its oral administration. Current GA treatments require regular intravitreal injections - procedures that are invasive, require specialist administration, and create substantial treatment burden for predominantly elderly patients. A once-daily oral medication could dramatically improve treatment accessibility and adherence if efficacy and safety are confirmed.
Several important limitations must be considered. The MAGNIFY study was concluded after enrolling only ~40% of planned participants (100 of 246), with just two-thirds completing 12 months of treatment and only one-third reaching the 18-month endpoint. The press release omits critical information regarding statistical significance, p-values, comprehensive safety data, and importantly, the reason for early study termination.
The cross-indication potential of zervimesine - now showing positive signals across Alzheimer's disease, dementia with Lewy bodies, and GA - suggests a mechanism potentially targeting common pathological processes in neurodegeneration. This could indicate broader therapeutic applications than current complement-focused GA treatments.
While these topline results appear encouraging, comprehensive evaluation requires the full dataset including safety profiles, visual function outcomes, and detailed statistical analysis. The GA market represents a substantial opportunity with millions of affected patients worldwide and limited treatment options, but zervimesine would need to demonstrate consistent efficacy and safety in larger confirmatory trials before potentially transforming the treatment paradigm in this vision-threatening disease.
Zervimesine treatment slowed the rate of GA lesion growth by
Observed GA lesion area was reduced by
Dry AMD results validate zervimesine’s potential across degenerative disease indications
PURCHASE, N.Y., May 08, 2025 (GLOBE NEWSWIRE) -- Cognition Therapeutics, Inc. (NASDAQ: CGTX), a clinical-stage company developing drugs that treat neurodegenerative disorders, reported topline results today from the Phase 2 COG2201 ‘MAGNIFY’ trial of zervimesine (CT1812) in adults with geographic atrophy (GA) secondary to dry age-related macular degeneration (dry AMD). The results show zervimesine-treated participants had
“To date, we have observed evidence of robust slowing of disease progression with zervimesine treatment in Phase 2 studies in Alzheimer’s disease and dementia with Lewy bodies,” explained Anthony O. Caggiano, MD, PhD, Cognition Therapeutics’ chief medical officer and head of R&D. “These results in dry AMD represent yet another indication in which zervimesine has potential to slow the progression of disease with a once-daily oral pill. Compared to current treatment options, which require regular clinic visits for intravitreal injections, an effective oral treatment that patients can take at home would be truly transformative.”
GA is characterized by the formation of lesions composed of dead retinal cells and undegraded waste proteins, creating blind spots in central vision. The change in GA lesions was measured using two methods: growth rate and size.
- Growth rate: in MAGNIFY, a slope analysis showed that the trajectory of GA had slowed by
28.6% in participants treated with zervimesine. - Size: at 18 months, the mean lesion size for zervimesine-treated participants was
28.2% smaller than placebo-treated. See Figure 1.
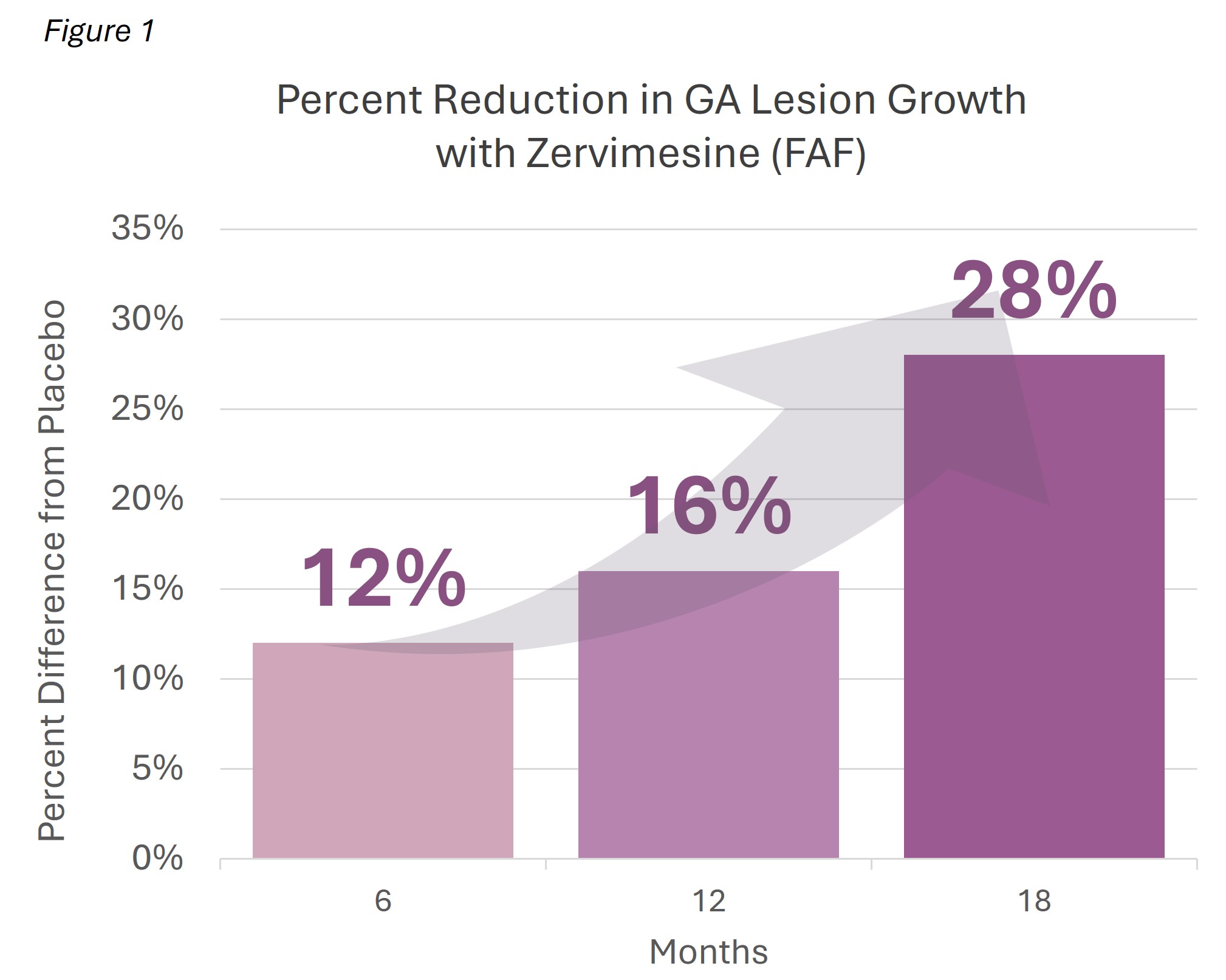
We believe these topline results compare favorably to published data from approved injectable complement inhibitors.
Approximately 2/3 of patients completed 12 months of dosing and 1/3 completed 18 months of dosing.
Additional data, including safety, demographics, and visual and anatomic outcomes are still being analyzed and will be reported at a later date. In addition, Cognition plans to submit complete findings for presentation at a medical meeting later this year.
“Dry AMD is now the third indication in which we have shown efficacy signals with a once-daily oral drug,” added Lisa Ricciardi, Cognition Therapeutics’ president and CEO. “We believe zervimesine has the potential to be used as a monotherapy or in combination with existing medications. This would allow physicians the flexibility to tailor treatment regimens for their patients. Importantly it would also allow patients who are not appropriate for injectables to have access to treatment. With the right partner and development plan, we believe zervimesine could be a treatment breakthrough in these large, underserved diseases.”
About the MAGNIFY Study (COG2201)
The MAGNIFY study was a double-masked, placebo-controlled Phase 2 clinical trial designed to enroll 246 adults with geographic atrophy (GA) secondary to dry age-related macular degeneration (dry AMD). Participants were evenly randomized to receive either placebo or 200 mg of once-daily oral zervimesine. The MAGNIFY study was concluded after approximately 100 of the planned 246 participants were enrolled. Participants in MAGNIFY were assessed for safety and tolerability, changes in GA lesion size and growth rate, changes in visual acuity and other anatomic and visual measures.
The decision to voluntarily conclude the MAGNIFY study prior to its intended enrollment goal has allowed the Company to focus resources on its ongoing programs in Alzheimer’s and DLB.
About Geographic Atrophy Secondary to Dry AMD
Dry AMD is the more prevalent of two forms of age-related macular degeneration and accounts for up to
About Zervimesine (CT1812)
Zervimesine (CT1812) is an investigational oral, once-daily pill being developed for the treatment of CNS diseases such as Alzheimer’s disease and dementia with Lewy bodies (DLB). While these diseases have different symptoms, both are associated with the buildup of certain proteins in the brain - Aβ and ɑ-synuclein. As these proteins bind to neurons, they can damage and ultimately destroy the neurons. This results in a progressive loss in a person’s ability to learn, recall memories, move efficiently, or communicate. These diseases progress relentlessly and ultimately result in death. If zervimesine can interrupt the toxic effects of these proteins, it may be able to slow progression of disease and improve the lives of those suffering from Alzheimer’s and DLB. Zervimesine has been generally well tolerated in clinical studies to date.
The USAN Council has adopted zervimesine as the United States Adopted Name (USAN) for CT1812.
About Cognition Therapeutics, Inc.
Cognition Therapeutics, Inc., is a clinical-stage biopharmaceutical company discovering and developing innovative, small molecule therapeutics targeting age-related degenerative disorders of the central nervous system. We are currently investigating our lead candidate, zervimesine (CT1812), in clinical programs in dementia with Lewy bodies (DLB) and Alzheimer’s disease, including the ongoing START study (NCT05531656) in early Alzheimer’s disease. We believe zervimesine can regulate pathways that are impaired in these diseases though its interaction with the sigma-2 receptor, a mechanism that is functionally distinct from other approaches for the treatment of degenerative diseases. More about Cognition Therapeutics and our pipeline can be found at https://cogrx.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. All statements contained in this press release, other than statements of historical facts or statements that relate to present facts or current conditions, including but not limited to, statements regarding our expected runway, product candidates, including zervimesine (CT1812), and any expected or implied benefits or results, including that initial clinical results observed with respect to zervimesine will be replicated in later trials and our clinical development plans, and expectations regarding timing, success and data announcements of current ongoing clinical trials are forward-looking statements. These statements, including statements relating to the timing and expected results of our clinical trials involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance, or achievements to be materially different from any future results, performance, or achievements expressed or implied by the forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “might,” “will,” “should,” “expect,” “plan,” “aim,” “seek,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “forecast,” “potential” or “continue” or the negative of these terms or other similar expressions. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our business, financial condition, and results of operations. These forward-looking statements speak only as of the date of this press release and are subject to a number of risks, uncertainties and assumptions, some of which cannot be predicted or quantified and some of which are beyond our control. Factors that may cause actual results to differ materially from current expectations include, but are not limited to: competition; our ability to secure new (and retain existing) grant funding; our ability to grow and manage growth, maintain relationships with suppliers and retain our management and key employees; our ability to successfully advance our current and future product candidates through development activities, preclinical studies and clinical trials and costs related thereto; uncertainties inherent in the results of preliminary data, pre-clinical studies and earlier-stage clinical trials being predictive of the results of early or later-stage clinical trials; the timing, scope and likelihood of regulatory filings and approvals, including regulatory approval of our product candidates; changes in applicable laws or regulations; the possibility that the we may be adversely affected by other economic, business or competitive factors, including ongoing economic uncertainty; our estimates of expenses and profitability; the evolution of the markets in which we compete; our ability to implement our strategic initiatives and continue to innovate our existing products; our ability to defend our intellectual property; impacts of global political changes and global economic conditions on our business, supply chain and labor force; our ability to maintain the listing of our common stock on the Nasdaq Global Market; and the risks and uncertainties described more fully in the “Risk Factors” section of our annual and quarterly reports filed with the Securities Exchange Commission and are available at www.sec.gov. These risks are not exhaustive, and we face both known and unknown risks. You should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur, and actual results could differ materially from those projected in the forward-looking statements. Moreover, we operate in a dynamic industry and economy. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties that we may face. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.
| Contact Information: Cognition Therapeutics, Inc. info@cogrx.com | Casey McDonald (media) Tiberend Strategic Advisors, Inc. cmcdonald@tiberend.com | Mike Moyer (investors) LifeSci Advisors mmoyer@lifesciadvisors.com |
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/d62491cf-a8af-4386-87f7-a6f4b98c2c4f
This press release was published by a CLEAR® Verified individual.








