Channel Therapeutics Announces Positive Efficacy Results for the Treatment of Eye Pain Using its NaV1.7 Inhibitor in Multiple Preclinical In Vivo Models
Rhea-AI Summary
Channel Therapeutics (NYSE: CHRO) has announced positive results from two preclinical animal trials for its eye drop formulation CT2000, targeting both acute and chronic ocular pain. The first trial demonstrated CT2000's effectiveness in treating acute eye pain using a capsaicin model, showing significant pain reduction within 15 minutes. The second trial, using a dry eye disease model, showed cumulative efficacy over a 7-day treatment period.
The company's NaV1.7 inhibitor showed promising results in both trials, supporting its potential broad applications in pain treatment. The global chronic ocular pain market, valued at $7.2 billion in 2023, is projected to reach $12.44 billion by 2032, growing at a CAGR of 6.6%. The acute eye pain market is valued at $404 million. These successful preclinical results pave the way for Channel to initiate Phase 1/II human proof of concept trials.
Positive
- None.
Negative
- Still in preclinical stage, requiring extensive clinical trials before potential commercialization
- Results limited to animal models, human efficacy yet to be demonstrated
News Market Reaction
On the day this news was published, CHRO declined 0.93%, reflecting a mild negative market reaction.
Data tracked by StockTitan Argus on the day of publication.
Global Chronic Ocular Pain Market Valued at US
FREEHOLD, N.J., May 14, 2025 (GLOBE NEWSWIRE) -- Channel Therapeutics Corporation, (“Channel” or the “Company”), (NYSE American: CHRO), a pioneer in the development of non-opioid pain treatment therapeutics, today announced that it has achieved its predefined endpoints in two pre-clinical animal models of the Company’s eye drop formulations (“CT2000”) for the treatment of both acute ocular pain as well as chronic ocular surface pain commonly associated with dry eye disease.
“We are very pleased with the results of these animal efficacy studies, which adds to the Depot formulation study results announced in December 2024, demonstrating a viable path forward in treating both post-surgical pain and chronic eye pain,” stated Dr. Eric Lang, Chief Medical Officer of Channel. “Additionally, these results support our belief that the inhibition of NaV1.7 has broad applications in treating different pain indications and further supports the genetic validation that NaV1.7 is a potent target for pain,” concluded Dr. Lang.
About the Trials / Results
Trial One
In the first trial, rabbits were treated with capsaicin (i.e., Pepper spray) to mimic an acute ocular insult in a common, validated model for acute eye pain studies. Following the capsaicin treatment, the rabbits were treated with CT2000, which was dosed four times over a 24-hour period. Pain was measured by the number of paw wipes over 60 seconds (paw wipes are a recognized surrogate of eye pain in animal models). The results showed that CT2000 significantly reduced the number of paw wipes within 15 minutes of administration of capsaicin and that CT2000 continued to show efficacy over a 60-minute period following administration. This eye pain model was only validated for a short duration, with the results summarized in the following graph:

Trial Two
In the second trial, benzalkonium chloride (“BAC”) was instilled in mice eyes over a multiday period to create a model of dry eye disease (the study was repeated twice). BAC is a detergent that irritates the eyes and simulates dry eye disease. As with the capsaicin model summarized above, increased paw wipes over 60 seconds were a surrogate to measure ocular pain. Following the induction of dry eye using BAC, the mice were dosed with CT2000 four times per day for 7 days. CT2000 reduced the frequency of paw wipes within a single day of administration and showed cumulative efficacy over time (the analgesic effect appeared to further improve when dosed over several days). The results after one day of dosing CT2000 are summarized in the following graph:
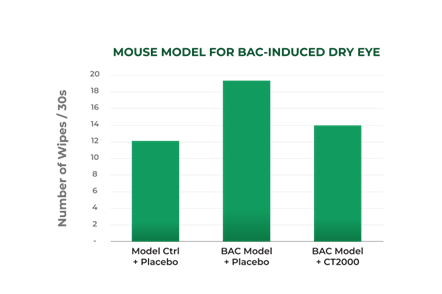
“We are very encouraged with these results, and the opportunity to target a large market that currently does not have many therapeutic options could provide Channel with considerable market opportunities,” stated Frank Knuettel II, Chief Executive Officer of Channel. “Moreover, these results pave the way for Channel to launch Phase 1/II human proof of concept trials,” Mr. Knuettel concluded.
According to Astute Analytica, the global annual chronic eye pain market was valued at
About Channel
Channel Therapeutics Corporation is a clinical-stage biotechnology company focused on developing and commercializing novel, non-opioid, non-addictive therapeutics to alleviate pain. The Company’s initial clinical focus is to selectively target the sodium ion-channel known as NaV1.7 for the treatment of various types of systemic chronic pain, acute and chronic eye pain and post-surgical nerve blocks. For company updates and to learn more about Channel, visit www.channeltherapeutics.com or follow us on social media.
Forward-Looking Statements
This press release contains forward-looking statements regarding the Company’s current expectations. These forward-looking statements include, without limitation, references to the Company’s expectations regarding (i) the Company’s belief that the results of its eye pain study, combined with the recently announced results of its Depot formulation demonstrate a viable path forward in treating both post-surgical pain and eye pain, (ii) the Company’s beliefs that the inhibition of NaV1.7 has broad applications in treating different pain indications and that NAV1.7 is a potent target for pain, (iii) the Company’s belief that the Company may be able to target a large market that currently does not have many therapeutic options, which could provide Channel with considerable market opportunities, and (iv) the launch of Phase 1/II human proof of concepts trials and the expected results of those human trials. These statements are not guarantees of future performance and are subject to certain risks, uncertainties and assumptions that are difficult to predict. Factors that could cause actual results to differ materially from those set forth in such forward-looking statements include, but are not limited to, risks and uncertainties related to there being no guarantee that the trading price of the Company’s Common Stock will be indicative of the Company’s value or that the Company’s Common Stock will become an attractive investment in the future. These and other risks and uncertainties are described more fully in in our filings with the U.S. Securities and Exchange Commission. The information in this press release is provided only as of the date of this press release, and we undertake no obligation to update any forward-looking statements contained in this press release based on new information, future events, or otherwise, except as required by law.
Channel Media and Investor Inquires:
For Investor Inquiries:
Mike Moyer
Managing Director, LifeSci Advisors, LLC
mmoyer@lifesciadvisors.com
Bar charts accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/7301f4d2-dd3b-4822-91cb-6f69833dc865
https://www.globenewswire.com/NewsRoom/AttachmentNg/898c17bd-7683-4e4f-954f-9e0c257ff161








