Novasight™ Hybrid Imaging System Featured in Published JSCAI Case Report Demonstrating Superior Diagnosis and Precision-Guided Therapy in Complex Coronary Case
Rhea-AI Summary
Conavi Medical (OTCQB: CNVIF) announced the publication of a significant case report in the Journal of the Society for Cardiovascular Angiography & Interventions featuring their Novasight™ Hybrid IVUS-OCT System. The case report demonstrates how the system's dual imaging capabilities led to superior diagnosis and treatment in a complex coronary case.
The study highlighted how the system correctly identified a plaque rupture that was initially misdiagnosed as calcification through traditional angiography. The technology combines high-resolution OCT imaging with IVUS anatomical reference points, enabling precise stent placement. CEO Thomas Looby confirmed the company's next-generation Novasight™ Hybrid System is on track for FDA 510(k) submission in Q3 2025.
Positive
- Clinical validation of Novasight™ Hybrid System's superior diagnostic capabilities in complex coronary cases
- FDA 510(k) submission for next-generation system on track for Q3 2025
- System aligns with new U.S. imaging Class 1A Guidelines for intracoronary imaging
- Technology addresses key adoption barriers in interventional cardiology
Negative
- None.
News Market Reaction
On the day this news was published, CNVIF declined 3.23%, reflecting a moderate negative market reaction.
Data tracked by StockTitan Argus on the day of publication.
TORONTO, July 30, 2025 (GLOBE NEWSWIRE) -- Conavi Medical Inc. (TSXV: CNVI) (OTCQB: CNVIF), a leader in hybrid intravascular imaging, is pleased to announce the publication of a compelling case report in the Journal of the Society for Cardiovascular Angiography & Interventions (JSCAI) highlighting the clinical impact of the Company’s Novasight™ Hybrid IVUS-OCT System in guiding accurate diagnosis and optimal stent placement in a complex coronary artery case. The case was performed, and the images were collected using the first-generation Novasight Hybrid IVUS-OCT system.
“This publication validates our belief that hybrid imaging is the future of precision-guided interventional cardiology,” said Thomas Looby, CEO of Conavi Medical. “Our goal is to help physicians make faster, more accurate decisions and ultimately improve patient outcomes. Also, this article is based on the first-generation system. We are especially excited about our next-generation Novasight™ Hybrid System, which is designed to deliver enhanced image quality and functionality for even greater physician and patient benefit, and the U.S. FDA 510(k) submission is on track for Q3 2025.”
The report, titled “The Role of Comprehensive Hybrid Imaging in Identification of Plaque Rupture and Ostial Stent Placement: Case Report” (DOI: 10.1016/j.jscai.2025.103814), details the treatment of a 52-year-old male patient with multiple cardiovascular risk factors, including diabetes, hypertension, hyperlipidemia, and end-stage renal disease. Initial angiography suggested a calcified lesion at the ostium of the right coronary artery (RCA). However, hybrid imaging using the Novasight™ System, integrating both intravascular ultrasound (IVUS) and optical coherence tomography (OCT), revealed the true culprit: a plaque rupture, not significant calcification.
“Hybrid imaging has the power to transform how we diagnose and treat complex coronary disease,” said Dr. Megha Prasad, co-author of the study and interventional cardiologist. “In this patient, angiography alone could have potentially led us down the wrong path. The ability to combine the high-resolution structural detail of OCT with the anatomical reference points of IVUS in real time allowed us to correctly identify the plaque rupture and precisely treat the lesion, something neither modality could have done alone.”
The case demonstrates how the simultaneous use of intravascular ultrasound (IVUS) and optical coherence tomography (OCT) via the Novasight Hybrid catheter enabled physicians to:
- Correctly identify a plaque rupture previously mischaracterized as calcification by angiography alone
- Leverage OCT’s high-resolution imaging to visualize intraplaque hemorrhage and rupture cavity
- Use IVUS to mark and visualize the coronary ostium with precision, allowing for accurate stent placement and optimal expansion
The publication reinforces the Novasight™ System’s role in aligning with new U.S. imaging Class 1A Guidelines for intracoronary imaging and Acute Coronary Syndrome (ACS) that advocate for broader use of intracoronary imaging in PCI. Conavi’s hybrid solution uniquely addresses long-standing barriers to adoption, including the need for separate imaging systems and interpretation challenges, particularly among less experienced operators.
Figure 1: Before percutaneous coronary intervention
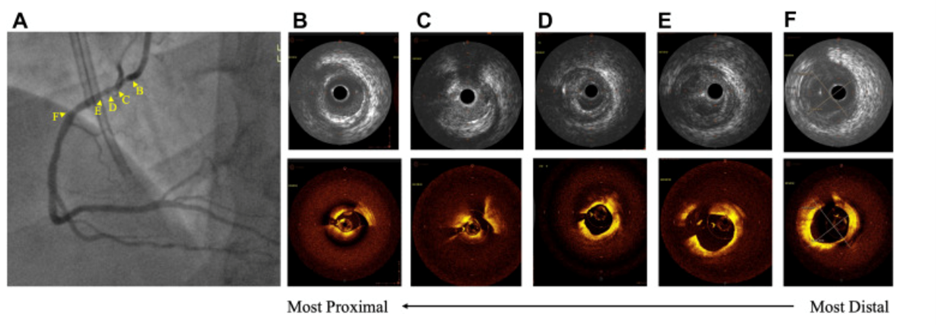
(A) Angiogram of right coronary artery (RCA) lesion; intravenous ultrasound (top) and optical coherence tomography (bottom) visualizations of (B) ostial RCA, (C) conus branch, (D) intraplaque hemorrhage and wire artifact, (E) plaque rupture cavity and wire artifact, and (F) distal reference
Figure 2: After percutaneous coronary intervention
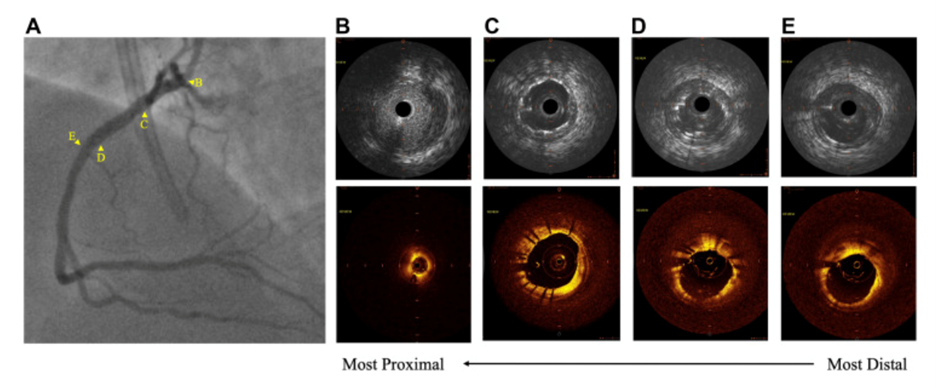
(A) Angiogram of right coronary artery (RCA) lesion; intravenous ultrasound (top) and optical coherence tomography (bottom) visualizations of (B) proximal edge of stent struts of ostial RCA (demonstrating no extension into the aorta), (C) minimal stent area of 9.40 mm2, (D) distal stent edge struts and wire artifact, and (E) the distal reference.
About Conavi Medical Corp.:
Conavi Medical is focused on designing, manufacturing, and marketing imaging technologies to guide common minimally invasive cardiovascular procedures. Its patented Novasight Hybrid™ System is the first system to combine both intravascular ultrasound (IVUS) and optical coherence tomography (OCT) to enable simultaneous and co-registered imaging of coronary arteries. The Novasight Hybrid System has 510(k) clearance from the U.S. Food and Drug Administration; and regulatory approval for clinical use from Health Canada, China's National Medical Products Administration, and Japan's Ministry of Health, Labor and Welfare. For more information, visit conavi.com.
Cautionary Statement Regarding Forward-Looking Information
This news release contains “forward-looking statements” within the meaning of applicable Canadian and U.S. securities laws, which reflect the current expectations of management of Conavi’s future growth, results of operations, performance and business prospects and opportunities. Forward-looking statements are frequently, but not always, identified by words such as “may”, “would”, “could”, “will”, “anticipate”, “believe”, “plan”, “expect”, “intend”, “estimate”, “potential for” and similar expressions, although these words may not be present in all forward-looking statements. Forward-looking statements that appear in this release may include, without limitation, references to Conavi’s plans for the commercialization of its Novasight Hybrid™ System.
These forward-looking statements reflect management’s current beliefs with respect to future events, and are based on information currently available to management that, while considered reasonable by management as of the date on which the statements are made, are inherently subject to significant business, economic and competitive uncertainties and contingencies which could result in actions, events, conditions, results, performance or achievements to be materially different from those projected in the forward-looking statements. Forward-looking statements involve significant risks, uncertainties and assumptions and many factors could cause Conavi’s actual results, performance or achievements to be materially different from any future results, performance or achievements that may be expressed or implied by such forward-looking statements. Such factors and assumptions include, but are not limited to, Conavi’s ability to retain key personnel; its ability to execute on its business plans and strategies; and other factors listed in the “Risk Factors” sections of the joint information circular of Conavi dated August 30, 2024 and in the final short form prospectus of Conavi dated April 15, 2025 (each of which may be viewed at www.sedarplus.com). Should one or more of these risks or uncertainties materialize, or should assumptions underlying the forward-looking statements prove incorrect, actual results, performance, or achievements may vary materially from those expressed or implied by the forward-looking statements contained in this news release. These factors should be considered carefully, and prospective investors should not place undue reliance on the forward-looking statements.
Although the forward-looking statements contained in the news release are based upon what management currently believes to be reasonable assumptions and Conavi has attempted to identify important factors that could cause actual actions, events, conditions, results, performance or achievements to differ materially from those described in forward-looking statements, Conavi cannot assure prospective investors that actual results, performance or achievements will be consistent with these forward-looking statements. Except as required by law, Conavi expressly disclaims any intention or obligation to update or revise any forward-looking statements whether as a result of new information, future events or otherwise. Accordingly, investors should not place undue reliance on forward-looking statements. All the forward-looking statements are expressly qualified by the foregoing cautionary statements.
Neither the TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this press release.
Contact
Stefano Picone
Chief Financial Officer
(416) 483-0100
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/a7e92599-d250-4dc4-b13d-cb919391c659
https://www.globenewswire.com/NewsRoom/AttachmentNg/f569ab74-cfbb-4716-8222-741370392d91








