Echo IQ Highlights Market Potential for EchoSolv AS Following Presentation of New Data for Severe Aortic Stenosis (AS) Presented at AHA Scientific Sessions 2025
Rhea-AI Summary
Echo IQ (OTC:ECHQF) highlighted two studies presented at AHA Scientific Sessions 2025 showing gaps in diagnosing and treating severe aortic stenosis (AS) and potential benefits of EchoSolv AS. A 30,878-echocardiogram investigator study found EchoSolv identified severe AS phenotypes more accurately than cardiologists and improved the male:female diagnosis ratio from 2.2:1 to 1.1:1. A separate real-world analysis of 1.18 million echocardiograms from NEDA linked to national health data found only 36% of women and 45% of men with moderate-to-severe AS received intervention and that “watchful waiting” was associated with substantial life-years lost. EchoSolv AS has FDA clearance and is positioned as a decision-support tool to reduce diagnostic delays and improve intervention timing.
Positive
- EchoSolv outperformed cardiologists in 30,878 echocardiograms
- Diagnosis gender ratio improved from 2.2:1 to 1.1:1
- NEDA analysis covered 1.18 million patients (2010–2021)
- EchoSolv AS has received FDA clearance
Negative
- Only 36% of women with moderate-to-severe AS received intervention
- Only 45% of men with moderate-to-severe AS received intervention
- ‘Watchful waiting’ linked to substantial life-years lost
News Market Reaction
On the day this news was published, ECHQF gained 14.41%, reflecting a significant positive market reaction.
Data tracked by StockTitan Argus on the day of publication.
- Investigator-initiated study shows potential for EchoSolv AS to drive improved patient outcomes when used to support cardiologist in identifying severe AS particularly in women
- NEDA study of 1.2M patients’ echocardiograms reveals gap in standard-of-care for severe AS; highlighting significant medical need for improved monitoring and diagnosis
SYDNEY, Nov. 12, 2025 (GLOBE NEWSWIRE) -- AI and Medical Technology company Echo IQ (“the Company” or “Echo IQ”) (ASX: EIQ) today announced that overwhelmingly convincing data from two recent studies have highlighted the significant gaps in the current standard-of-care for diagnosing and monitoring severe aortic stenosis (AS), and potential for EchoSolv AS to drive improved patient outcomes. The results from both studies were presented separately at the American Heart Association (AHA) Scientific Sessions 2025, which was held in New Orleans, Louisiana.
Chief Executive Officer, Mr Dustin Haines said: “The cumulative data from the two severe AS studies presented by the University of Notre Dame Australia Researchers at AHA25 have reinforced our commitment to leveraging advanced analytics and AI to transform severe AS care for patients. A key takeaway from the study that reviewed echocardiograms for more than 1.2 million patients is that the ‘watchful waiting’ strategy for moderate-to-severe AS cases is failing many of these patients. In addition, we are excited by the insights from the results that show how EchoSolv AS was able to identify the severe AS phenotype more accurately than cardiologists, particularly in some subtypes of AS. This study was of particular interest as it showed clearly that we have work to do in accurately diagnosing women with aortic stenosis and we are optimistic with the performance of EchoSolv AS to improve the gender equity.”
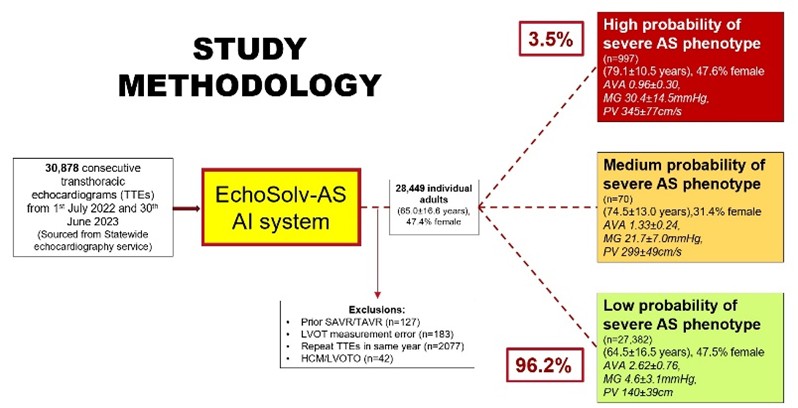
Study titled, “Accuracy of cardiologist reporting of severe aortic stenosis vs decision-support artificial intelligence and its impact on clinical management”
Presented by Dr. Vikas Bhat, University of Notre Dame Australia, this investigator-initiated study compared traditional cardiologist reporting with EchoSolv™, an AI-powered clinical decision support system. Findings from 30,878 echocardiograms revealed:
- EchoSolv outperformed cardiologists in identifying severe AS phenotypes, particularly in women and low-gradient cases.
- EchoSolv-driven reporting restored gender equity in diagnosis, with male to female ratio improved from 2.2:1 to 1.1:1.
- Adoption of real-time EchoSolv support could reduce delays in diagnosis and valve intervention, improving patient outcomes.
Echo Report Influences Follow-up and Management
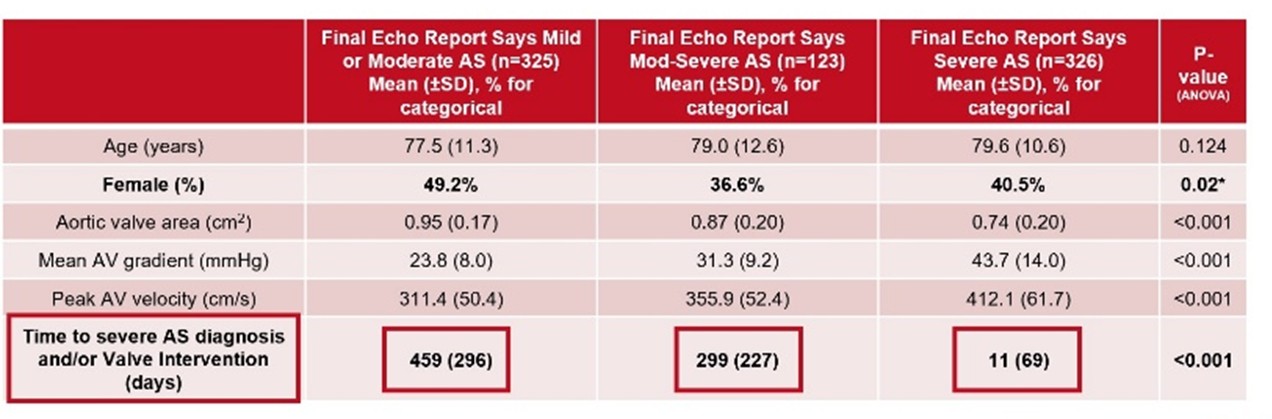
n=774 meeting severe AS guidelines
Only patients REPORTED severe were considered for valve intervention
This study is an investigator-initiated study and received no external funding.
Study titled, “Hemodynamic profile of Aortic Stenosis versus health service delivery from 1,200,000 individuals”
Presented by Professor Geoff Strange, Professor of Medicine at The University of Notre Dame Australia, this real-world included the analysis of 1.18 million subjects aged at least 18 years and undergoing echocardiography during the period of 2010 - 2021 for repeat or investigation of cardiovascular disease. These data were pulled from 45 centres in Australia that contribute to the National Echo Database of Australia (NEDA), and individuals were then linked to the Australian Institute of Health and Welfare’s (AIHW’s), National Health Data Hub (NHDH). The study suggests:
- A significant discordance between haemodynamic severity and treatment rates – only
36% of women and45% of men with moderate-to-severe AS received intervention. - “Watchful waiting” strategy linked to substantial life-years lost compared to timely aortic valve replacement (AVR).
- Findings underscore the need for policy and clinical practice changes to address treatment gaps and improve survival.
This study was funded by an IIS grant from Edwards LifeScience. The funders played no part in the data curation / analysis / preparation of these data.
EchoSolv AS is poised to change this paradigm by enabling more precise and consistent identification of high-risk AS patients, thereby supporting clinicians in making informed, timely decisions regarding intervention. Globally, AS is a widespread but frequently underdiagnosed condition, primarily caused by calcification of the aortic valve, which serves as a critical gateway to the heart. Failure to diagnose AS in a timely manner can lead to missed opportunities for lifesaving interventions, such as valve replacement surgery. EchoSolv AS has undergone rigorous validation, including FDA Clearance, through extensive testing across both the United States and Australia, demonstrating the utility of aiding in the accurate diagnosis of patients with aortic stenosis.
NEDA principal investigator, Professor David Playford, the senior author for both studies, said: “These studies confirm previous reports in two ways. First, we reaffirm that moderate and severe aortic stenosis is a life threatening condition that causes significant health service utilization, heart failure hospitalizations and premature mortality. Second, artificial intelligence is capable of outperforming cardiologists in the identification of the set of abnormalities that happen when aortic stenosis progresses toward the severe state. These two findings, taken together, reinforce that EchoSolv AS AI should be considered as a routine option during echocardiographic reporting.
Authorised for release by the Board of Directors of Echo IQ Limited.
| Investor Enquiries: | |
| Andrew Grover, Executive Chair | Henry Jordan, Six Degrees Investor Relations |
| Andrew.grover@echoiq.ai / investor@echoiq.ai | Henry.jordan@sdir.com.au / +61 (0) 431 271 538 |
ABOUT ECHO IQ
Echo IQ uses AI-driven technology and proprietary software to improve decision making in Cardiology.
The company is based in Sydney, Australia.
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/2edccc84-6a52-409f-a130-b402f1f62f43
https://www.globenewswire.com/NewsRoom/AttachmentNg/cb55c68a-d320-456b-a219-7e11b54dc98d








