Immuron Submits IMM-529 IND to FDA
Rhea-AI Summary
Immuron (NASDAQ: IMRN) submitted an IND to the FDA for clinical development of IMM-529 to treat Clostridioides difficile infection (CDI) and prevent recurrent CDI (rCDI). The company plans to initiate a Phase 2 trial in H1 2026 enrolling first-episode and recurrent CDI patients. An independent market analysis estimates an eligible population of ~98,000 patients at first recurrence and a base-case annual revenue potential of ~US$400M pending efficacy. IMM-529 is an oral, three-target polyclonal antibody candidate directed at Toxin B, spores, and surface layer proteins, with preclinical results reporting prevention of primary disease (80% P=0.0052), protection of recurrence (67% P<0.01) and treatment of primary disease (78.6% P<0.0001).
Positive
- IND submission to FDA for IMM-529 targeting CDI
- Phase 2 clinical trial planned to start in H1 2026
- Oral administration favored by infectious disease specialists
- Preclinical efficacy: primary prevention 80% (P=0.0052)
- Preclinical efficacy: recurrence protection 67% (P<0.01)
- Preclinical efficacy: treatment effect 78.6% (P<0.0001)
- Independent market base-case revenue estimate ~US$400M
Negative
- Phase 2 trial not started until H1 2026, delaying human readouts
- Revenue projection (~US$400M) is contingent on demonstrated clinical efficacy
- Estimated eligible population (~98,000) assumes use at first recurrence per payer positioning
News Market Reaction – IMRN
On the day this news was published, IMRN gained 2.71%, reflecting a moderate positive market reaction. Argus tracked a peak move of +2.8% during that session. Argus tracked a trough of -19.4% from its starting point during tracking. Our momentum scanner triggered 2 alerts that day, indicating moderate trading interest and price volatility. This price movement added approximately $391K to the company's valuation, bringing the market cap to $15M at that time.
Data tracked by StockTitan Argus on the day of publication.
Key Points
- Immuron submits Investigational new drug (IND) application to FDA for clinical development of IMM-529 as product to specifically prevent or treat Clostridioides difficile infection (CDI)
- Previous clinical trial data on IMM-529 provides support for continued development of IMM-529
MELBOURNE, Australia, Oct. 08, 2025 (GLOBE NEWSWIRE) -- Immuron Limited (ASX: IMC; NASDAQ: IMRN), is pleased to announce that it has submitted an Investigational New Drug (IND) application to the United States Food and Drug Administration (FDA) for clinical development of IMM-529. Under this new IND, Immuron is proposing the development of IMM-529 for treatment of Clostridioides difficile (C. diff) infection (CDI) and prevention of recurrent CDI (rCDI).
The Company plans to initiate a Phase 2 clinical trial for IMM-529 in individuals with Clostridioides difficile infection (CDI) during the first half of 2026.
Independent Market analysis conducted by Lumanity suggests that, pending demonstration of efficacy, IMM-529 could be positioned at the earliest point in the treatment algorithm permitted by payer guidelines. The Phase 2 clinical trial is expected to enroll both first episode and recurrent CDI patients. The estimated eligible population of patients would be approximately 98,000 individuals if IMM-529 is introduced as a treatment at the first recurrence stage.
Considering market size, anticipated payer dynamics, competitive landscape, and pricing assumptions, base case annual revenue potential for IMM-529 is projected at approximately US
The increased incidence of antibiotic resistant ‘superbugs’ has amplified the use of broad-spectrum antibiotics worldwide. An unintended consequence of antimicrobial treatment is disruption of the gastrointestinal microbiota, resulting in susceptibility to opportunistic pathogens, such as Clostridioides difficile (C. diff). Paradoxically, treatment of Clostridioides difficile infection (CDI) also involves antibiotic use, and the heavy reliance on antibiotics to control C. diff does not allow for the gut flora to regenerate and predisposes the patient to relapsing CDI. C. diff is currently the most common pathogen in healthcare-associated infections and was deemed an urgent threat in the Center for Disease Control and Prevention’s report on antibiotic resistance threats in the United States (CDC, 2019). CDI affects over 400,000 people in the US on a yearly basis, contributing to over 30,000 deaths in the US alone annually. This serious health threat has led to an urgent call for the development of new therapeutics to reduce or replace the use of antibiotics to treat bacterial infections.
To address this need, Immuron is developing IMM-529 as an adjunctive therapy in combination with standard of care antibiotics for the treatment of CDI and prevention recurrent CDI. IMM-529 has been designed with antibodies that target the key essential virulence components of C. diff, with the potential to accelerate the clearance of CDI infection and support rapid restoration of healthy gut microbiota. This mechanism of action positions IMM-529 as a compelling oral therapeutic drug candidate for the treatment of CDI and preventative of disease recurrent CDI, addressing a critical unmet need in infectious disease management. Immuron is collaborating with Dr. Dena Lyras and her team at Monash University, Australia to develop vaccines designed to elicit antibodies against essential C. diff virulence components. IMM-529 specifically targets Toxin B (TcB), the spores, and the surface layer proteins of the vegetative cells (refer to MOA schematic - below).
This novel 3-target approach has yielded promising results in pre-clinical infection and relapse models, including (1) Prevention of primary disease (
To our knowledge, IMM-529 is, to date, the only investigational drug that has shown therapeutic potential in all three phases of the disease. https://doi.org/10.1038/s41598-017-03982-5
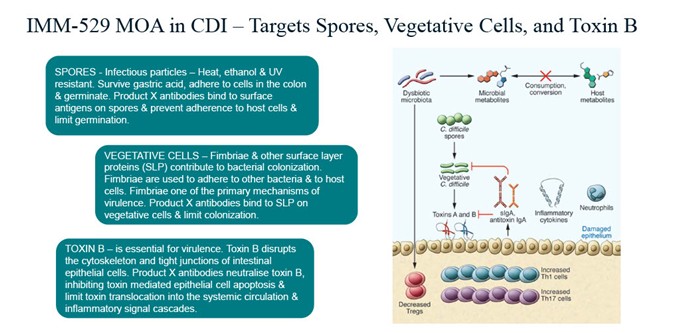
Figure: Mechanism of Action of IMM-529 targeting Clostridioides difficile spores, cells and toxin B
This release has been authorised by the directors of Immuron Limited.
COMPANY CONTACT:
Steven Lydeamore
Chief Executive Officer
steve@immuron.com
About Immuron
Immuron Limited (ASX: IMC, NASDAQ: IMRN), is an Australian biopharmaceutical company focused on developing and commercializing orally delivered targeted polyclonal antibodies for the treatment of infectious diseases.
Immuron Platform Technology
Immuron’s proprietary technology is based on polyclonal immunoglobulins (IgG) derived from engineered hyper-immune bovine colostrum. Immuron has the capability of producing highly specific immunoglobulins to any enteric pathogen and our products are orally active. Bovine IgG can withstand the acidic environment of the stomach and is resistant to proteolysis by the digestive enzymes found in the Gastrointestinal (GI) tract. Bovine IgG also possesses this unique ability to remain active in the human GI tract delivering its full benefits directly to the bacteria found there. The underlying nature of Immuron’s platform technology enables the development of medicines across a large range of infectious diseases. The platform can be used to block viruses or bacteria at mucosal surfaces such as the Gastrointestinal tract and neutralize the toxins they produce.
For more information visit: https://www.immuron.com.au/ and https://www.travelan.com
Sign up to Immuron’s Investor Hub: Here
FORWARD-LOOKING STATEMENTS:
This press release may contain “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, each as amended. Such statements include, but are not limited to, any statements relating to our growth strategy and product development programs and any other statements that are not historical facts. Forward-looking statements are based on management’s current expectations and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition, and stock value. Factors that could cause actual results to differ materially from those currently anticipated include: risks relating to our growth strategy; our ability to obtain, perform under and maintain financing and strategic agreements and relationships; risks relating to the results of research and development activities; risks relating to the timing of starting and completing clinical trials; uncertainties relating to preclinical and clinical testing; our dependence on third-party suppliers; our ability to attract, integrate and retain key personnel; the early stage of products under development; our need for substantial additional funds; government regulation; patent and intellectual property matters; competition; as well as other risks described in our SEC filings. We expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in our expectations or any changes in events, conditions, or circumstances on which any such statement is based, except as required by law.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/0f651b8e-0c29-4151-bb9a-32be85b26697








