Immuneering Reports Positive Overall Survival Data for Atebimetinib (IMM-1-104) from Ongoing Phase 2a Trial in First-Line Pancreatic Cancer Patients
Rhea-AI Summary
Positive
- Exceptional 94% overall survival rate at 6 months, significantly higher than 67% standard of care benchmark
- Strong 72% progression-free survival at 6 months versus 44% standard benchmark
- Impressive 39% overall response rate and 81% disease control rate with durable tumor regressions
- Superior tolerability profile with minimal Grade 3+ adverse events
- Planned advancement to pivotal trial in 2026 indicates strong confidence in results
Negative
- Median overall survival and progression-free survival not yet reached, requiring longer follow-up
- Pivotal trial won't begin until 2026, indicating a lengthy timeline to potential approval
- Limited patient sample size of 34-36 patients in current trial data
News Market Reaction 1 Alert
On the day this news was published, IMRX declined 23.21%, reflecting a significant negative market reaction.
Data tracked by StockTitan Argus on the day of publication.
-
- Striking tumor reductions with
- Markedly favorable tolerability profile observed, demonstrating potential best-in-class profile -
- Pivotal trial of atebimetinib in combination with mGnP in first-line pancreatic cancer patients planned for 2026 -
- Company to host conference call at 8:00 a.m. ET today -
CAMBRIDGE, Mass., June 17, 2025 (GLOBE NEWSWIRE) -- Immuneering Corporation (Nasdaq: IMRX), a clinical-stage oncology company outpacing cancer to help patients outlive their disease, today announced positive data from its ongoing Phase 2a clinical trial evaluating atebimetinib (IMM-1-104), an oral, once-daily novel MEK inhibitor, in combination with modified gemcitabine/nab-paclitaxel (mGnP) in first-line pancreatic cancer patients.
“These exceptional data demonstrate the potential of atebimetinib plus mGnP to dramatically extend the lives of patients with advanced pancreatic cancer,” said Ben Zeskind, Ph.D., Co-founder and Chief Executive Officer of Immuneering. “
Durability and Tolerability of Atebimetinib + mGnP Demonstrated in Phase 2a Data
94% overall survival (OS) was observed at 6 months in first-line (1L) pancreatic cancer patients treated with atebimetinib + mGnP at the 320 mg once-daily dose of atebimetinib (N=34). The benchmark 6-month OS for the standard of care treatment in this population (full dose and schedule GnP) is67% .1 The median OS was not yet reached at the data cutoff date.72% progression-free survival (PFS) was observed at 6 months in first-line (1L) pancreatic cancer patients treated with atebimetinib + mGnP at the 320 mg dose level (N=34). The benchmark 6-month PFS for the standard of care treatment in this population (full dose and schedule GnP) is44% .1 The median PFS was not yet reached at the data cutoff date.- An overall response rate (ORR) of
39% and a disease control rate (DCR) of81% were observed in response evaluable patients at both the 240 and 320 mg dose levels of atebimetinib + mGnP (N=36), including many patients with deepening, durable regressions and multiple examples of individual lesions rendered undetectable. - Atebimetinib continued to demonstrate a markedly favorable tolerability profile in combination with mGnP. No Grade 3+ events were observed in a majority of the adverse event categories commonly observed with standard of care chemotherapy in first line pancreatic cancer.
- Based on these data, the Company has increased target enrollment in the 1L pancreatic cancer atebimetinib + mGnP combination arm to approximately 50 patients.
- All results are reported using a data cutoff date of May 26, 2025.
1Von Hoff et al, 2013 NEJM
OS and PFS for Atebimetinib (320 mg QD) + mGnP (N=34, Intent-to-Treat Population)
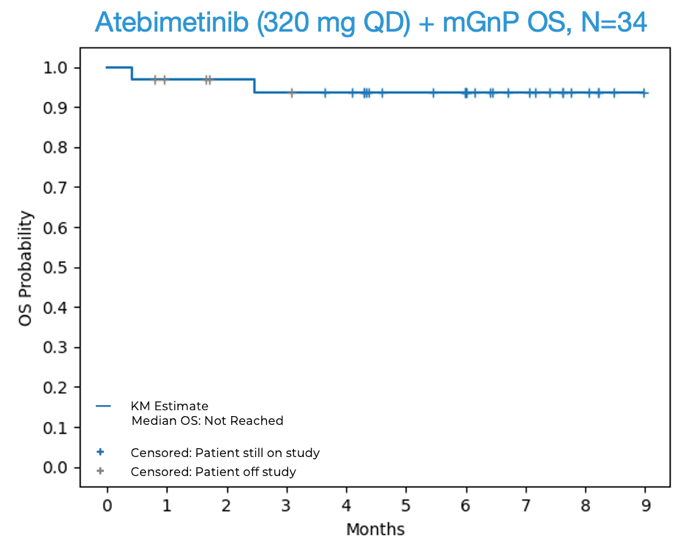
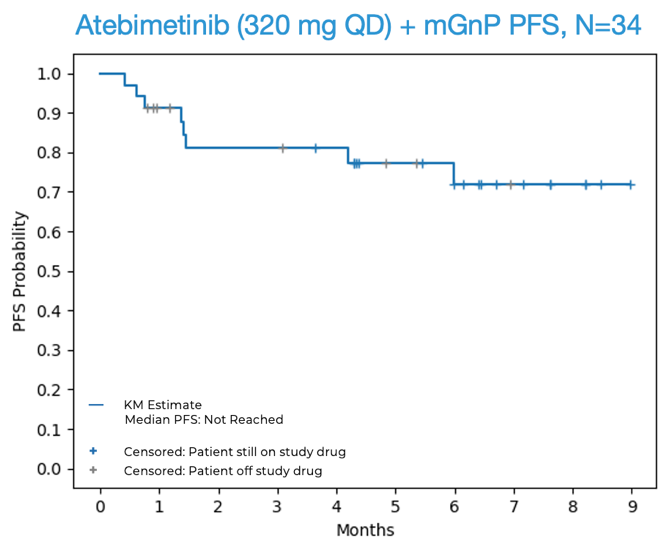
Source: Immuneering Corporation.
“The encouraging clinical data reported thus far for atebimetinib (IMM-1-104) represent a potential new and significantly more durable treatment option for pancreatic cancer patients, for whom limited therapeutic options are currently available,” said Vincent Chung, M.D., F.A.C.P., Professor, Department of Medical Oncology and Therapeutics Research at City of Hope, and principal investigator of the Phase 2a clinical trial. “I have treated pancreatic cancer patients with atebimetinib who have experienced exceptional durability. Current standard of care therapies in pancreatic cancer can be associated with limited durability and severe side effects, leading to poor patient outcomes. We have not seen significant improvement in standard of care for decades, and there is an urgent need for more durable and better tolerated new treatments that help patients live longer.”
“These data clearly establish atebimetinib’s potential as a more durable and better tolerated MEK inhibitor positioned to help patients both live longer and live better, with exciting potential opportunities in pancreatic cancer and a variety of other cancers,” said Igor Matushansky, M.D. Ph.D., Chief Medical Officer of Immuneering. “We look forward to advancing atebimetinib to a pivotal trial as rapidly as possible.”
Dr. Chung is a paid advisory board member of Immuneering.
Near-Term Milestone Expectations
Building on these new Phase 2a clinical data, Immuneering is planning for several additional milestones related to atebimetinib, including:
- Regulatory feedback on pivotal study plans in 4Q 2025
- Data from additional patients in Phase 2a trial in 4Q 2025
- Initiation of pivotal, randomized trial of atebimetinib in combination with mGnP in first-line pancreatic cancer in 2026
- Initiation of additional atebimetinib clinical trial combination arms in 2026
Conference Call
Immuneering will host a conference call and live webcast at 8:00 a.m. ET / 5:00 a.m. PT on June 17, 2025, to discuss the data and provide a business update. Individuals interested in listening to the live conference call may do so by dialing (800) 715-9871 for U.S callers and (646) 307-1963 for other locations and reference conference ID 5641694, or from the webcast link in the “investors” section of the company's website at www.immuneering.com. A webcast replay will be available in the investor relations section on the company’s website for 90 days following the completion of the call.
About Immuneering Corporation
Immuneering is a clinical-stage oncology company outpacing cancer to help patients outlive their disease. The Company’s lead product candidate, atebimetinib (IMM-1-104), is an oral, once-daily deep cyclic inhibitor of MEK designed to improve durability and tolerability, and expand indications to include MAPK pathway-driven tumors such as most pancreatic cancers. Atebimetinib is currently in a Phase 2a trial in patients with advanced solid tumors including pancreatic cancer. The Company’s development pipeline also includes early-stage programs. For more information, please visit www.immuneering.com.
Forward-Looking Statements
This press release contains forward-looking statements, including within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including, without limitation, statements regarding: our plans to develop, manufacture and commercialize our product candidates; the treatment potential of atebimetinib, alone or in combination with other agents, including modified Gemcitabine/nab-paclitaxel (mGnP); the plans and objectives of Company management for future operations, including with respect to the timing, planning and execution of enrollment, additional atebimetinib combination trials and a potential pivotal trial of atebimetinib in combination with mGnP; the timing for release of additional results from the Phase 2a portion of the trial for atebimetinib; the timing and substance of regulatory feedback on pivotal study plans; and expectations regarding our cash runway.
These forward-looking statements are based on management’s current expectations. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: the risks inherent in oncology drug research and development, including target discovery, target validation, lead compound identification, and lead compound optimization; we have incurred significant losses, are not currently profitable and may never become profitable; our projected cash runway; our need for additional funding and ability to continue as a going concern; our unproven approach to therapeutic intervention; our ability to address regulatory questions and the uncertainties relating to regulatory filings, reviews and approvals; the lengthy, expensive, and uncertain process of clinical drug development, including potential delays in or failure to obtain regulatory approvals; our reliance on third parties and collaborators to conduct our clinical trials, manufacture our product candidates, and develop and commercialize our product candidates, if approved; failure to compete successfully against other drug companies; protection of our proprietary technology and the confidentiality of our trade secrets; potential lawsuits for, or claims of, infringement of third-party intellectual property or challenges to the ownership of our intellectual property; our patents being found invalid or unenforceable; costs and resources of operating as a public company; and unfavorable or no analyst research or reports.
These and other important factors discussed under the caption “Risk Factors” in our Quarterly Report on Form 10-Q for the three months ended March 31, 2025, and our other reports filed with the U.S. Securities and Exchange Commission, could cause actual results to differ materially from those indicated by the forward-looking statements made in this press release. Any such forward-looking statements represent management's estimates as of the date of this press release. While we may elect to update such forward-looking statements at some point in the future, except as required by law, we disclaim any obligation to do so, even if subsequent events cause our views to change. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this press release.
Media Contact:
Jenna Urban
212-253-8881
jurban@cglife.com
Investor Contact:
Laurence Watts
619-916-7620
laurence@newstreetir.com
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/ed73ce19-d09c-47f0-aad1-3d3b3b4f6dc5
https://www.globenewswire.com/NewsRoom/AttachmentNg/2a74ffea-0ae9-4221-9a43-ee5bd266d124








