Lunai Bioworks achieves complete regression of both primary and metastatic pancreatic tumors in preclinical humanized models, marking a breakthrough in allogeneic cancer immunotherapy
Rhea-AI Summary
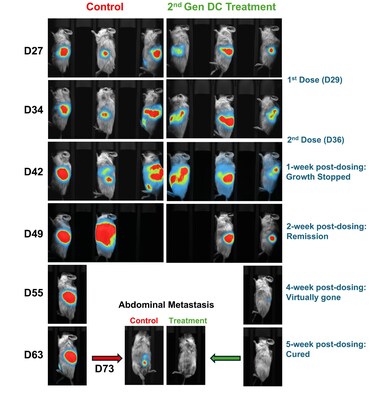
Lunai Bioworks (NASDAQ: LNAI) announced that a second‑generation, clinical‑grade dendritic cell (DC) therapy produced complete regression of primary and metastatic pancreatic tumors in preclinical humanized mouse models.
The Brief Report was published in Vaccines on Nov 2, 2025 and describes an optimized, clinically compliant construct that preserves the original product's efficacy while improving promoter and manufacturing elements to support IND‑enabling studies.
The therapy triggered robust activation of cytotoxic T cells and NK cells, and the company says the data advance partnering conversations and the platform's translation toward clinical development.
Positive
- Complete regression of primary and metastatic tumors in humanized mouse models
- Published in Vaccines on Nov 2, 2025
- Second‑generation construct preserved efficacy while improving clinical design
- Therapy triggered robust activation of cytotoxic T cells and NK cells
Negative
- Findings limited to preclinical humanized mouse models; no human data yet
- No IND filing or clinical trial readout reported in the announcement
News Market Reaction
On the day this news was published, LNAI gained 40.29%, reflecting a significant positive market reaction. Argus tracked a peak move of +38.0% during that session. Argus tracked a trough of -11.5% from its starting point during tracking. Our momentum scanner triggered 45 alerts that day, indicating elevated trading interest and price volatility. This price movement added approximately $10M to the company's valuation, bringing the market cap to $35M at that time. Trading volume was exceptionally heavy at 990.4x the daily average, suggesting very strong buying interest.
Data tracked by StockTitan Argus on the day of publication.
Lunai Bioworks Announces Publication of Second-Generation Allogeneic Dendritic Cell Therapy Platform in the journal "Vaccines".
The study was published in a new Brief Report in Vaccines on November 2, 2025. The peer-reviewed Brief Report details the development of a second-generation, clinical-grade DC construct that achieved complete regression of both primary and metastatic pancreatic tumors in preclinical humanized models. Pancreatic cancer is one of the deadliest cancers, often diagnosed late and resistant to conventional treatments. Lunai's therapy uses engineered dendritic cells — specialized immune cells created from stem cells — that are genetically enhanced to activate the body's immune system against cancer. This publication details a second-generation, clinical-compliant version of this platform, with the provided evidence critical in advancing ongoing partnering conversations for the therapy.
This new publication builds on Lunai's earlier study (Vaccines 2025 Jul 12;13(7):749; doi:10.3390/vaccines13070749), which first demonstrated potent anti-tumor activity of CD34+ hematopoietic stem cell (HSC)-derived DCs engineered to overexpress CD40L, CD93, and CXCL13 in humanized mouse models of pancreatic cancer. While the initial study utilized a research-grade lentiviral vector, the latest report describes an optimized, clinically compliant construct that preserves equivalent efficacy while improving design and manufacturing attributes to support future clinical translation.
The second-generation construct features a strong mammalian promoter and enhanced genetic elements to drive expression of key immune-modulating molecules. In preclinical humanized models, Lunai's next-generation DC therapy triggered robust activation of cytotoxic T cells and NK cells and led to complete regression of both primary and metastatic pancreatic tumors. These findings mirrored the outcomes achieved with the original research-grade product. While the allogeneic DC product has been evaluated in pancreatic cancer models, its therapeutic potential extends to a broad range of solid tumors.
"These findings demonstrate that our vector optimization strategy maintains therapeutic potency while bringing our allogeneic DC platform to the threshold of IND-enabling studies," said David Weinstein, CEO of Lunai Bioworks. "This represents an important inflection point as we move from research innovation to clinical translation—positioning Lunai at the forefront of next-generation cell-based immunotherapies."
The full article, titled "Modified Hematopoietic Stem Cell-Derived Dendritic Cell Therapy Retained Tumor-Inhibitory Function and Led to Regression of Primary and Metastatic Pancreatic Tumors in Humanized Mouse Models," is available in Vaccines (https://www.mdpi.com/2076-393X/13/11/1131).
About Lunai
Lunai Bioworks Inc. is an AI-powered drug discovery and biodefense company pioneering safe and responsible generative biology. With proprietary neurotoxicity datasets, advanced machine learning, and a focus on dual-use risk management, Lunai is redefining how artificial intelligence can accelerate therapeutic innovation while safeguarding society from emerging threats. For more visit www.lunaibioworks.com.
Forward-Looking Statements:
This press release contains "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995, including statements regarding future operations, plans, and market expectations. These statements are based on current assumptions and beliefs and are subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied. Words such as "may," "will," "expect," "believe," "anticipate," "intend," "plan," "estimate," and similar expressions are intended to identify forward-looking statements. Actual results may differ due to factors beyond the Company's control. You should not place undue reliance on these statements, which speak only as of the date of this release. For additional information about risks and uncertainties that could affect the Company, please refer to its filings with the Securities and Exchange Commission at www.sec.gov. The Company undertakes no obligation to update or revise any forward-looking statements, except as required by law.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/lunai-bioworks-achieves-complete-regression-of-both-primary-and-metastatic-pancreatic-tumors-in-preclinical-humanized-models-marking-a-breakthrough-in-allogeneic-cancer-immunotherapy-302605740.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/lunai-bioworks-achieves-complete-regression-of-both-primary-and-metastatic-pancreatic-tumors-in-preclinical-humanized-models-marking-a-breakthrough-in-allogeneic-cancer-immunotherapy-302605740.html
SOURCE Lunai Bioworks Inc.