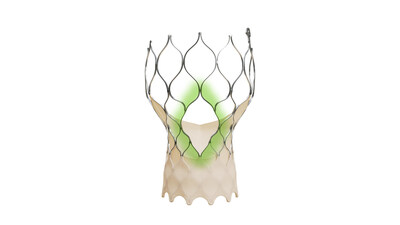
Medtronic announces FDA approval of newest-generation Evolut TAVR system for treatment of symptomatic severe aortic stenosis
The Evolut™ FX+ TAVR system leverages market-leading valve performance with addition of larger windows to facilitate coronary access
The Evolut FX+ TAVR system offers larger coronary access windows through a modified diamond-shaped frame design, which is four times larger than previous iterations of the Evolut TAVR system. Evolut FX+ provides increased space for catheter maneuverability to facilitate access to coronary arteries of varying patient anatomies. Additionally, the new design does not compromise the market leading valve performance, excellent hemodynamics, and radial strength that clinicians expect from the Evolut platform.1
"We are committed to consistently developing and advancing minimally invasive solutions for physicians to treat their patients with aortic stenosis. This is reinforced by our continued innovation of the Evolut TAVR platform, which has delivered proven valve performance and durability to physicians and patients for years. The Evolut FX+ TAVR system was designed to facilitate coronary access across a diverse range of patient anatomies with no compromise to valve performance," said Jeffrey Popma, M.D., vice president and chief medical officer for the Coronary & Renal Denervation business and the Structural Heart & Aortic business, which are part of the Cardiovascular Portfolio at Medtronic.
Severe aortic stenosis occurs when the aortic valve leaflets become stiff and thickened and have difficulty opening and closing, making the heart work harder to pump blood to the rest of the body. Severe aortic stenosis often reduces a patient's quality of life and limits their daily activities. If left untreated,
The Evolut FX+ TAVR system is indicated for symptomatic severe aortic stenosis patients across all risk categories (extreme, high, intermediate, and low) in the
About Medtronic
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Dublin,
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
1Medtronic data on file compared to the Evolut platform. Bench top model may not be indicative of clinical performance.
2Ross J Jr, Braunwald E. Aortic stenosis. Circulation. July 1968; 38(1 Suppl):61-67.
Contacts: | |
Rachel Murray | Ryan Weispfenning |
Public Relations | Investor Relations |
+1-651-332-3286 | +1-763-505-4626 |
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/medtronic-announces-fda-approval-of-newest-generation-evolut-tavr-system-for-treatment-of-symptomatic-severe-aortic-stenosis-302100338.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/medtronic-announces-fda-approval-of-newest-generation-evolut-tavr-system-for-treatment-of-symptomatic-severe-aortic-stenosis-302100338.html
SOURCE Medtronic plc