MyMD Pharmaceuticals Announces New Data from Comparative Study on Lead Compound MYMD-1
MyMD Pharmaceuticals, Inc. (“MyMD”), a clinical stage pharmaceutical company committed to extending healthy lifespan by focusing on developing two therapeutic platforms, today announced new data from a study conducted by Eurofins Discovery (ERF.FP) Phenotypic Center of Excellence (“Eurofins”) comparing the biological activities of MyMD’s lead compound MYMD-1, with leading FDA approved TNF inhibitors (TNFi).
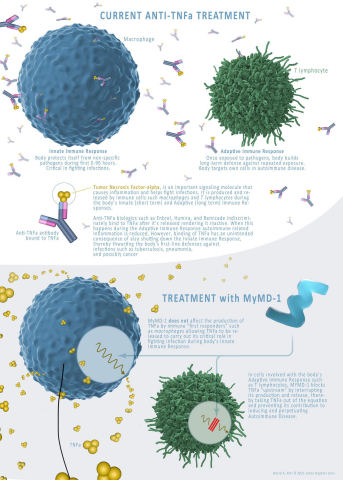
MYMD-1 is being developed to treat autoimmune and age-related diseases, and to slow aging thereby increasing life-span. The drug has shown effectiveness in regulating the immune system, in preclinical studies, by targeting the root causes of inflammation and performing as a selective inhibitor of tumor necrosis factor-alpha (TNF-a), a driver of chronic inflammation.
The recent Eurofins study found that when compared to commercially available TNFi biological drugs, MYMD-1 had significant activity demonstrating anti-proliferative effects – the effect of inhibiting cell growth. Data also demonstrated that none of the biological drugs selected for the comparison study proved to be anti-proliferative to any of the primary cell types assessed.
“This data continues to validate our concerted efforts to move forward in bringing MYMD-1 to the market and its promising potential to treat a myriad of inflammatory and autoimmune-related diseases,” said Chris Chapman, M.D., President, Director and Chief Medical Officer of MyMD. “We remain encouraged as we continue our evaluations of MYMD-1 for efficacy and safety – especially as studies continue to suggest we may be able to address many of the concerns that are associated with the current drugs on the market today in these disease areas.”
The current FDA approved TNFi biological drugs selected for the comparative study treat a number of inflammatory and autoimmune diseases, including Crohn's disease, ulcerative colitis, rheumatoid arthritis, psoriasis, psoriatic arthritis, and more. Acumen Research and Consulting confirmed TNF-α inhibitors are the most prescribed drugs by revenue globally, at
“Current TNF-a inhibitors available today, while effective, come with an array of adverse effects and concerns for patients,” said Adam Kaplin, M.D., Ph.D, Chief Scientific Officer for MyMD. “Many have the risk of causing neurotoxicity, as they are unable to cross the blood–brain barrier; this is one of the key differentiators and capabilities of MYMD-1, which crosses the blood-brain barrier. We also found that MYMD-1’s selectivity allows it to only block overactive TNF-a in lymphocytes that participate in autoimmune diseases, leaving TNF-a synthesized in macrophages to be produced to help coordinate the initial response to acute infections. This should remove the increased risk of infection, associated with all TNFi biological drugs used today.”
“The power of the BioMAP Platform lies in the ability to compare a compound’s biological profile, or fingerprint, against a large reference database of profiles from approved drugs and tool compounds,” said Alison O’Mahony, Ph.D., Vice President of Translational Biology at Eurofins Discovery. “These advanced analytics provide a human-centric, data-driven approach to phenotypic discovery for clients.”
The study was completed using the BioMAP® Diversity PLUS® Panel for broad phenotypic profiling and screening from Eurofins Discovery. The BioMAP Diversity PLUS Panel provides biologically relevant in vitro models of human disease and is used to analyze drug candidate compounds, from discovery to preclinical safety. BioMAP Diversity PLUS systems are made of human primary cell-based assays modeling complex tissue and disease biology of organs. BioMAP Diversity PLUS informs a drug candidate’s potency, selectivity, safety, mechanism of action and disease indication. Additionally, these findings can provide actionable insights to help progress the right molecules to further clinical testing.
MyMD will continue conducting studies with Eurofins to determine the efficacy of MYMD-1 and its key differentiators when compared to current drugs on the market. These efforts continue to support MyMD’s efforts to move forward in Phase 2 clinical studies.
About MyMD Pharmaceuticals, Inc.
MyMD is a clinical stage pharmaceutical company committed to extending healthy lifespan by focusing on developing two therapeutic platforms. MYMD-1 is a drug platform based on a clinical stage small molecule that regulates the immunometabolic system to control TNF-α and other pro-inflammatory cytokines. MYMD-1 is being developed to treat autoimmune diseases, including those currently treated with non-selective TNF-α blocking drugs, and aging and longevity. Supera-CBD is a drug platform based on a novel (patent pending) synthetic derivative of cannabidiol (CBD) that targets numerous key receptors including CB2 and opioid receptors and inhibits monoamine oxidase. Supera-CBD is being developed to address the rapidly growing CBD market, that includes FDA approved drugs and CBD products not currently regulated as a drug. For more information, visit www.mymd.com.
About Eurofins – the global leader in bio-analysis
Eurofins is testing for life. Eurofins is a global leader in food, environment, and pharmaceutical product testing. It is also one of the market leaders in testing and laboratory services for genomics, discovery pharmacology, forensics, advanced material sciences and has a rapidly developing presence in highly specialised and molecular clinical diagnostic testing.
With over 50,000 staff across a network of more than 800 laboratories in over 50 countries, Eurofins’ companies offer a portfolio of over 200,000 analytical methods.
Eurofins Shares are listed on Euronext Paris Stock Exchange.
Cautionary Statement Regarding Forward-Looking Statements
This press release may contain forward-looking statements. These forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause actual results, performance or achievements to be materially different from any expected future results, performance, or achievements. Forward-looking statements speak only as of the date they are made and none of MyMD nor its affiliates assume any duty to update forward-looking statements. Words such as "anticipate," "believe," "could," "estimate," "expect," "may," "plan," "will," "would'' and other similar expressions are intended to identify these forward-looking statements. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements include, without limitation: the timing of, and MyMD’s ability to, obtain and maintain regulatory approvals for clinical trials of MyMD’s pharmaceutical candidates; the timing and results of MyMD’s planned clinical trials for its pharmaceutical candidates; the amount of funds MyMD requires for its pharmaceutical candidates; increased levels of competition; changes in political, economic or regulatory conditions generally and in the markets in which MyMD operates; MyMD’s ability to retain and attract senior management and other key employees; MyMD’s ability to quickly and effectively respond to new technological developments; MyMD’s ability to protect its trade secrets or other proprietary rights, operate without infringing upon the proprietary rights of others and prevent others from infringing on MyMD’s proprietary rights; and the impact of the ongoing COVID-19 pandemic on MyMD’s results of operations, business plan and the global economy. A discussion of these and other factors with respect to MyMD is set forth in the Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2021, filed by MyMD on May 18, 2021. Forward-looking statements speak only as of the date they are made and MyMD disclaims any intention or obligation to revise any forward-looking statements, whether as a result of new information, future events or otherwise.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210707005497/en/







