Nexalin Technology Unveils Next-Generation HALO™ Clarity
A new neurostimulation device to combat the growing global mental health epidemic
Designed for drug-free treatment of mental health conditions from the
privacy of one’s home with virtual monitoring by a physician
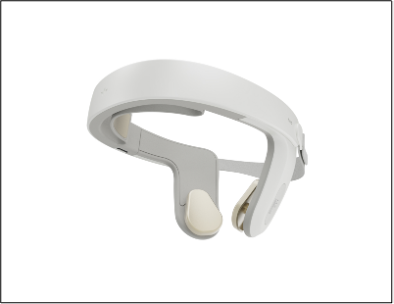
Photo: HALO™ Clarity headset
HOUSTON, TEXAS, Jan. 11, 2024 (GLOBE NEWSWIRE) -- Nexalin Technology, Inc. (the “Company” or “Nexalin”) (Nasdaq: NXL; NXLIW) today unveiled its Gen-3 HALO™ Clarity 15 milliamp (mA) neurostimulation device designed to treat a variety of mental health conditions, including Major Depressive Disorder (MDD), addiction and substance use disorder, Alzheimer’s disease, traumatic brain injury (TBI), post-traumatic stress disorder (PTSD), chronic pain and other potentially-applicable stress-related neuropsychiatric disorders.
The HALO™ Clarity utilizes an advanced technique, Deep Intracranial Frequency Stimulation (DIFS™), to penetrate structures deep in the mid-brain that are associated with mental health disorders. Nexalin believes that this innovative technique in its next-generation devices will generate enhanced patient therapeutic responses without adverse side effects. The HALO™ Clarity device has been the subject of multiple clinical trials in China whose findings were published in medical journals. In these published clinical studies, the resulting data shows a substantial and statistically significant benefit to patients.
Nexalin believes that the HALO™ Clarity represents a significant leap forward over prior versions of the device in multiple respects: it enables patients to receive treatment in the privacy of their own homes, and provides physicians with remote access to seamlessly monitor their patient data through a proprietary medical app which uses a secure electronic method of transmission. The HALO™ Clarity, designed for maximum comfort and convenience, has completed usability and electrical testing by the China National Medical Products Administration with positive results.
Nexalin plans to conduct clinical trials of the HALO™ Clarity in the U.S. and is in the process of consulting with the U.S. Food and Drug Administration (FDA) as part of its pre-submission meetings. The Company expects that its upcoming clinical trials will be completed in a quicker timeframe and at a considerably lower expense, due to the fact that the HALO™ Clarity treatment can be administered at home, as opposed to a hospital or outpatient clinical setting, and the resulting data can be captured, and patient response, contemporaneously transmitted electronically. In addition, and contingent upon FDA approval, the home-use aspect of HALO™ Clarity is expected to significantly reduce patient treatment costs, while increasing compliance with applicable standards.
Mark White, CEO of Nexalin Technology, stated, “The HALO™ Clarity marks a significant advance and possibly the most meaningful event in the history of the Company. When we established Nexalin and embarked on this journey, we set out to develop a first-in-class neurostimulation device, superior to anything currently available in the market. The HALO™ Clarity represents the culmination of years of research, testing and product development. I truly believe this device will revolutionize how we treat mental health disorders in the United States and around the world.”
“We approached the market from both the physician and patient perspective. Our goal has been to build an entire digital ecosystem to address inefficiencies in the market and stigmas associated with mental health disorders. Not only does our device build on extensive clinical data demonstrating the potential therapeutic effect of our prior generation devices, but it adds a whole new level of functionality, enabling treatment from the comfort and convenience of one’s own home, as well as remote monitoring by a physician. We anticipate that the ability to remotely treat and monitor patients will significantly reduce the financial and economic burden of mental healthcare.”
“Overall, we are clearly executing on our mission to transform the paradigm of mental health treatment, while reducing the growing dependence on drugs that are often ineffective and may have undesirable side effects. We could not be more excited about the outlook for the business and I look forward to providing further updates.”
About Nexalin Technology, Inc.
Nexalin designs and develops innovative neurostimulation products to uniquely and effectively help combat the ongoing global mental health epidemic. All of Nexalin’s products are non-invasive and undetectable to the human body and developed to provide relief to those afflicted with mental health issues. Nexalin utilizes bioelectronic medical technology to treat mental health issues. Nexalin believes its neurostimulation medical devices can penetrate structures deep in the mid-brain that are associated with mental health disorders. Nexalin believes the deeper penetrating waveform in its next-generation devices will generate enhanced patient response without any adverse side effects. The Nexalin Gen-2 15 milliamp (mA) neurostimulation device has been approved in China by the National Medical Products Administration (NMPA) for the treatment of insomnia and depression. Additional information about the Company is available at: https://nexalin.com/.
FORWARD-LOOKING STATEMENTS
This press release contains statements that constitute "forward-looking statements," These statements relate to future events or Nexalin’s future financial performance. Any statements that refer to expectations, projections or other characterizations of future events or circumstances or that are not statements of historical fact (including without limitation statements to the effect that Nexalin or its management “believes”, “expects”, “anticipates”, “plans”, “intends” and similar expressions) should be considered forward looking statements that involve risks and uncertainties which could cause actual events or Nexalin’s actual results to differ materially from those indicated by the forward-looking statements. Forward-looking statements are subject to numerous conditions, many of which are beyond the control of the Company, including those set forth in the Risk Factors section of the Company's Report on Form 10-K for the year ended December 31, 2022 and other filings as filed with the Securities and Exchange Commission. Copies of such filings are available on the SEC's website, www.sec.gov. Such forward-looking statements are made as of the date hereof and may become outdated over time. Such forward-looking statements are made as of the date hereof and may become outdated over time. The Company undertakes no obligation to update these statements for revisions or changes after the date of this release, except as required by law.
Contact:
Crescendo Communications, LLC
Tel: (212) 671-1020
Email: NXL@crescendo-ir.com
Attachment








