Radiopharm Theranostics Reports Preclinical Lu177-B7H3-mAb Data Demonstrating Favourable Biodistribution and High Tumour Uptake
Rhea-AI Summary
Positive
- Favorable preclinical data showing high tumor uptake and effective targeting
- Shorter half-life (1-2 days vs 1+ week) potentially reducing toxicity risks
- Liver excretion pathway offers advantages over kidney elimination
- Previous studies demonstrated complete tumor regression and immune system stimulation
- IND submission and Phase 1 trial initiation timeline confirmed for 2025
Negative
- Still in preclinical stage with no human trial data yet
- Phase 1 trials won't begin until Q4 2025
- Potential competition from other B7-H3 targeted therapies in development
News Market Reaction
On the day this news was published, RADX gained 11.16%, reflecting a significant positive market reaction.
Data tracked by StockTitan Argus on the day of publication.
Supports plans to advance the RV01 program to first-in-human therapeutic basket study in solid tumour cancers
Data completes preclinical package for Investigational New Drug submission in mid-2025 with Phase 1 therapeutic study initiation expected in 4Q25
SYDNEY, Australia, June 02, 2025 (GLOBE NEWSWIRE) -- Radiopharm Theranostics (ASX: RAD, Nasdaq: RADX, “Radiopharm” or the “Company”), a clinical-stage biopharmaceutical company focused on developing innovative oncology radiopharmaceuticals for areas of high unmet medical need, today announced that preclinical data from studies with the Lu177-B7H3-monoclonal antibody RV01 demonstrated favourable biodistribution and showed that RV01 maintained high tumour uptake. In addition, the Fc region modifications of the monoclonal antibody (mAb) confirm a shorter half-life, compared to traditional mAbs. The shorter half-life and other Fc modifications have the potential to limit off-target exposure to the isotope and mitigate toxicities associated with traditional monoclonal antibody therapies.
RV01 is the Company’s B7-H3-targeted radiopharmaceutical therapy designed with strong affinity for the 4Ig isoform of B7H3 that is highly expressed in tumours and not in healthy tissues, which is being developed in partnership with MD Anderson Cancer Center. The B7-H3 targeting monoclonal antibody (mAb) is designed to target various solid tumours that express the B7-H3 protein. High expression of this target is associated with a poor prognosis in many cancer types.
“We are especially pleased with these new preclinical data as they further validate earlier preclinical work that showed strong affinity to the target without the extensive circulation time of other monoclonal antibodies. Our mAb has been purposefully modified in the Fc-region with the objective of maintaining the same targeting capabilities together with a reduced half-life. Most mAbs have a typical half-life of over one week, whereas RV01 peaks within one to two days,” stated Dimitris Voliotis, M.D., Chief Medical Officer of Radiopharm Theranostics.
“The antibody shows faster liver excretion in the preclinical experiments, allowing the isotope enough time to effectively target the tumor potentially without the associated toxicities. Unlike peptides or small molecules, mAbs are excreted by the liver, the most radio-resistant organ. This, combined with the faster excretion due to the shortened half-life, potentially offers important advantages compared to excretion via the kidneys, where the elimination of radiopharmaceuticals can result in significant potential toxicities,” added Dr. Voliotis.
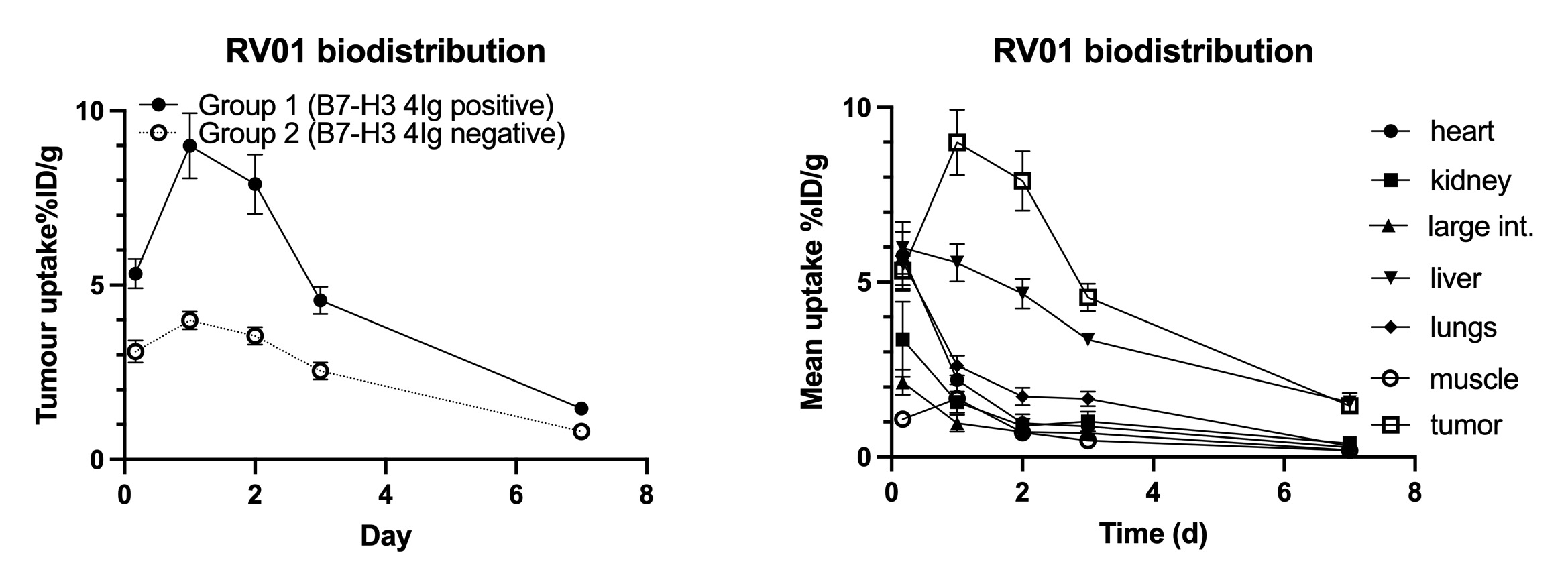
The new preclinical data corroborate conclusions from earlier preclinical mouse studies that identified a high-affinity antibody highly selective to the cancer-specific 4Ig isoform of the B7-H3 receptor, demonstrated complete regression of established solid tumours with treatment and showed promising evidence of immune system stimulation and the ability to confer immune memory.
“This biodistribution study is the last preclinical work needed to complete the preclinical package for our Investigational New Drug Application (IND) with the U.S. Food and Drug Administration. These data allow us to confirm our guidance to file the IND-submission in mid-2025 and to initiate our first-in-human Phase 1 basket study by the end of this year,” said Riccardo Canevari, CEO and Managing Director of Radiopharm Theranostics.
About Radiopharm Theranostics
Radiopharm Theranostics is a clinical stage radiotherapeutics company developing a world-class platform of innovative radiopharmaceutical products for diagnostic and therapeutic applications in areas of high unmet medical need. Radiopharm is listed on ASX (RAD) and on NASDAQ (RADX). The company has a pipeline of distinct and highly differentiated platform technologies spanning peptides, small molecules and monoclonal antibodies for use in cancer. The clinical program includes one Phase 2 and three Phase 1 trials in a variety of solid tumor cancers including lung, breast, and brain. Learn more at radiopharmtheranostics.com.
Authorized on behalf of the Radiopharm Theranostics Board of Directors by Executive Chairman Paul Hopper.
For more information:
Investors:
Riccardo Canevari
CEO & Managing Director
P: +1 862 309 0293
E: rc@radiopharmtheranostics.com
Anne Marie Fields
Precision AQ (formerly Stern IR)
E: annemarie.fields@precisionaq.com
Media:
Matt Wright
NWR Communications
P: +61 451 896 420
E: matt@nwrcommunications.com.au
Follow Radiopharm Theranostics:
Website – https://radiopharmtheranostics.com/
X – https://x.com/TeamRadiopharm
LinkedIn – https://www.linkedin.com/company/radiopharm-theranostics/
InvestorHub – https://investorhub.radiopharmtheranostics.com/
An image accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/7ff2e681-52ea-43e1-9cf4-250f2bb12480








