REGENXBIO Highlights Key 2026 Catalysts and Announces Positive Long-Term Functional Outcomes in Lead Duchenne Gene Therapy Program
Rhea-AI Summary
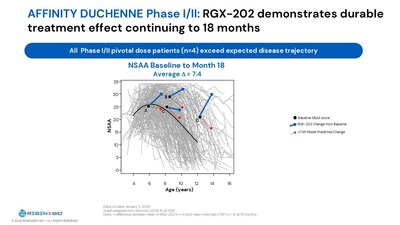
REGENXBIO (Nasdaq: RGNX) reported positive long-term Phase I/II RGX-202 functional data for Duchenne, with treated patients improving an average of 7.4 NSAA points versus cTAP at 18 months (n=4) and 6.6 points at 12 months. The company expects pivotal topline data in early Q2 2026 and plans a mid-2026 BLA filing under accelerated approval while continuing confirmatory enrollment. REGENXBIO also highlighted an FDA PDUFA date of Feb 8, 2026 for RGX-121, a $100M AbbVie milestone tied to sura-vec first patient dosing, and in-house manufacturing readiness from its Rockville facility.
Positive
- RGX-202 showed +7.4 NSAA points vs cTAP at 18 months
- Pivotal topline data expected early Q2 2026
- Planned mid-2026 BLA submission under accelerated pathway
- FDA PDUFA date set for Feb 8, 2026 for RGX-121
- $100 million milestone from AbbVie upon first DR patient dosing
- Completed process performance qualification lots for RGX-202
Negative
- Regulatory approvals remain pending, including FDA decisions in 2026
- Confirmatory trial enrollment not complete at time of BLA filing
- Commercial launches contingent on multiple successful 2026 readouts
Key Figures
Market Reality Check
Peers on Argus
RGNX fell 2.73% while several gene therapy/biotech peers also traded lower (e.g., REPL -3.02%, ATYR -1.18%, ARCT -1.02%), with one notable gainer (KOD +2.51%). Scanner data flags this as stock-specific rather than a coordinated sector momentum move.
Historical Context
| Date | Event | Sentiment | Move | Catalyst |
|---|---|---|---|---|
| Dec 18 | Conference presentation | Neutral | +2.6% | Announcement of presentation at the 44th Annual J.P. Morgan Healthcare Conference. |
| Nov 25 | Investor conference | Neutral | +10.0% | Participation in Piper Sandler 37th Annual Healthcare Conference with fireside chat. |
| Nov 06 | Earnings report | Neutral | -3.8% | Q3 2025 financials plus timelines for RGX-202, RGX-121 PDUFA, and sura-vec trials. |
| Oct 30 | Clinical progress | Neutral | -0.2% | Completion of pivotal RGX-202 enrollment and initiation of commercial production. |
| Oct 29 | Earnings call notice | Neutral | -2.4% | Scheduling of Q3 2025 results conference call and webcast details. |
Recent company communications (conferences, enrollment updates, earnings) have shown mixed short-term price reactions without a clear pattern of consistent alignment or divergence.
Over the last several months, REGENXBIO has focused on advancing late-stage AAV gene therapy programs and investor visibility. It completed pivotal enrollment for RGX-202 in AFFINITY DUCHENNE (pivotal n=30) and moved toward commercial-scale production. Q3 2025 results showed revenue of $29.7M and cash of $302.0M as of Sept 30, 2025, with expectations to fund operations into early 2027. The current announcement extends this trajectory with longer-term Duchenne functional data and confirms 2026 regulatory and data milestones already telegraphed in past updates.
Regulatory & Risk Context
An effective S-3 shelf filed on 2025-11-26 allows REGENXBIO to offer up to $300,000,000 of various securities over time for general corporate purposes, including clinical development, manufacturing scale-up, and potential acquisitions. No usage has been recorded yet (usage_count 0), but the capacity provides flexibility for future capital raises.
Market Pulse Summary
This announcement underscores maturing late-stage assets, with RGX-202 showing durable 18‑month functional benefit and a pivotal readout expected in early Q2 2026, alongside an RGX-121 PDUFA date on February 8, 2026 and pivotal sura-vec data in Q4 2026. These milestones build on prior disclosures of completed pivotal enrollment and commercial manufacturing progress. Investors may track execution on trial timelines, regulatory interactions, and potential use of the $300,000,000 shelf as the company advances toward possible launches between 2026 and 2028.
Key Terms
phase i/ii medical
biologics license application (bla) regulatory
pdufa regulatory
priority review voucher (prv) regulatory
aav gene therapies medical
suprachoroidal delivery medical
subretinal delivery medical
ind readiness regulatory
AI-generated analysis. Not financial advice.
- New Phase I/II RGX-202 functional data demonstrates long-term, durable treatment effect at pivotal dose at 18 months
- Robust patient enrollment in confirmatory trial continues, expect majority of patients to be dosed by planned BLA filing, mid-year
- Expecting FDA PDUFA decision and multiple pivotal top-line data readouts in 2026 to support potential commercial launches 2026-2028
- In-house manufacturing and strategic global partnerships driving commercial readiness
- Presentation at 44th Annual J.P. Morgan Healthcare Conference Wednesday, January 14
"2026 is set to be a transformative year for REGENXBIO, as we enter commercial stage with two near-term catalysts from our three late-stage assets and a clear path to sustained growth," said Curran Simpson, President and CEO, REGENXBIO. "We are starting the year with exciting new long-term data for our Duchenne program, demonstrating how our comprehensive strategy to maximize the potential for therapeutic benefit across all our programs is resulting in positive outcomes for patients. We are continuing to set the bar high for how potentially life-changing gene therapies are discovered, developed, and manufactured; this year we are sharply focused on advancing our commercial readiness to enable successful launches of these medicines for patients in need.
CLINICAL PROGRAM UPDATES AND 2026 ANTICIPATED MILESTONES
RGX-202 for Duchenne Muscular Dystrophy
New Functional Data
- REGENXBIO today announced new, positive 18-month functional data from patients treated with the pivotal dose in the Phase I/II portion of the AFFINITY DUCHENNE® trial (n=4). All patients exceeded expected disease trajectory on the North Star Ambulatory Assessment (NSAA) using the established cTAP disease progression model. RGX-202 recipients improved an average of 7.4 points compared to cTAP. These same patients improved an average of 6.6 points compared to cTAP at 12 months post-treatment. The Company plans to share additional Phase I/II safety, biomarker, and functional data at the MDA Clinical and Scientific Conference in March 2026.
Clinical Trial and Regulatory Milestones
- REGENXBIO expects to share pivotal topline data in early Q2 2026 and submit a Biologics License Application (BLA) under the accelerated approval pathway in mid-2026. Following the completion of enrollment in the pivotal trial (n=30) in October 2025, the Company continues to enroll in the confirmatory trial and expects to have majority of this trial enrolled at the time of BLA filing.
- Regulatory interactions with the FDA and European Medical Association (EMA) are planned for 1H 2026, supporting the global expansion of the AFFINITY DUCHENNE® trial.
Clemidsogene lanparvovec (RGX-121) for MPS II, also known as Hunter syndrome
- FDA PDUFA target date is February 8, 2026. FDA approval would result in receipt of a Priority Review Voucher (PRV), to which REGENXBIO has full rights.
- Partner Nippon Shinyaku, with its
U.S. subsidiary NS Pharma, is prepared to commercialize clemidsogene lanparvovec following potential approval. REGENXBIO plans to lead the clinical and commercial manufacturing its in-house Manufacturing Innovation Center inRockville, Md.
Surabgene lomparvovec (sura-vec, ABBV-RGX-314) for wet age-related macular degeneration (wet AMD) and diabetic retinopathy (DR)
Sura-vec is being developed in collaboration with AbbVie, and could be the first gene therapy for a non-rare disease, if approved.
- Sura-vec is on track to be the first gene therapy for wet AMD. REGENXBIO expects top-line data from ATMOSPHERE® and ASCENT® pivotal trials of sura-vec using subretinal delivery in Q4 2026.
- REGENXBIO will initiate a two-part sham injection-controlled Phase IIb/III trial of sura-vec for DR using suprachoroidal delivery. The Company will receive a
$100 million
Leading Gene Therapy Capabilities
REGENXBIO is one of the only gene therapy companies with fully in-house, end-to-end capabilities from capsid engineering and discovery through commercial-ready manufacturing, designed to reliably scale supply and realize the blockbuster potential of its gene therapy portfolio. At the REGENXBIO Manufacturing Innovation Center, in
REGENXBIO continues to expand the therapeutic potential of AAV gene delivery through capsid discovery and engineering. The Company is approaching IND readiness for the treatment of geographic atrophy using a new capsid that has demonstrated higher transgene expression via suprachoroidal delivery to the eye.
J.P. Morgan Healthcare Conference Presentation
President and CEO Curran Simpson will present at the J.P Morgan Healthcare Conference on Wednesday, January 14, 2026 at 10:30 a.m. PT. A live webcast of the presentation can be accessed in the Investors section of REGENXBIO's website at www.regenxbio.com. An archived replay of the webcast will be available for approximately 30 days following the presentation.
ABOUT REGENXBIO Inc.
REGENXBIO is a biotechnology company on a mission to improve lives through the curative potential of gene therapy. Since its founding in 2009, REGENXBIO has pioneered the field of AAV gene therapy. REGENXBIO is advancing a late-stage pipeline of one-time treatments for rare and retinal diseases, including RGX-202 for the treatment of Duchenne; clemidsogene lanparvovec (RGX-121) for the treatment of MPS II and RGX-111 for the treatment of MPS I, both in partnership with Nippon Shinyaku; and surabgene lomparvovec (ABBV-RGX-314) for the treatment of wet AMD and diabetic retinopathy, in collaboration with AbbVie. Thousands of patients have been treated with REGENXBIO's AAV platform, including those receiving Novartis' ZOLGENSMA®. REGENXBIO's investigational gene therapies have the potential to change the way healthcare is delivered for millions of people. For more information, please visit www.REGENXBIO.com.
FORWARD-LOOKING STATEMENTS
This press release includes "forward-looking statements," within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These statements express a belief, expectation or intention and are generally accompanied by words that convey projected future events or outcomes such as "believe," "may," "will," "estimate," "continue," "anticipate," "assume," "design," "intend," "expect," "could," "plan," "potential," "predict," "seek," "should," "would" or by variations of such words or by similar expressions. The forward-looking statements include statements relating to, among other things, REGENXBIO's future operations, clinical trials, costs and cash flow. REGENXBIO has based these forward-looking statements on its current expectations and assumptions and analyses made by REGENXBIO in light of its experience and its perception of historical trends, current conditions and expected future developments, as well as other factors REGENXBIO believes are appropriate under the circumstances. However, whether actual results and developments will conform with REGENXBIO's expectations and predictions is subject to a number of risks and uncertainties, including the timing of enrollment, commencement and completion and the success of clinical trials conducted by REGENXBIO, its licensees and its partners, the timing of commencement and completion and the success of preclinical studies conducted by REGENXBIO and its development partners, the timing or likelihood of payments from AbbVie or Nippon Shinyaku, the monetization of any priority review voucher, the timely development and launch of new products, the ability to obtain and maintain regulatory approval of product candidates, the ability to obtain and maintain intellectual property protection for product candidates and technology, trends and challenges in the business and markets in which REGENXBIO operates, the size and growth of potential markets for product candidates and the ability to serve those markets, the rate and degree of acceptance of product candidates, and other factors, many of which are beyond the control of REGENXBIO. Refer to the "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" sections of REGENXBIO's Annual Report on Form 10-K for the year ended December 31, 2024, and comparable "risk factors" sections of REGENXBIO's Quarterly Reports on Form 10-Q and other filings, which have been filed with the SEC and are available on the SEC's website at WWW.SEC.GOV. All of the forward-looking statements made in this press release are expressly qualified by the cautionary statements contained or referred to herein. The actual results or developments anticipated may not be realized or, even if substantially realized, they may not have the expected consequences to or effects on REGENXBIO or its businesses or operations. Such statements are not guarantees of future performance and actual results or developments may differ materially from those projected in the forward-looking statements. Readers are cautioned not to rely too heavily on the forward-looking statements contained in this press release. These forward-looking statements speak only as of the date of this press release. Except as required by law, REGENXBIO does not undertake any obligation, and specifically declines any obligation, to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Zolgensma® is a registered trademark of Novartis Gene Therapies. All other trademarks referenced herein are registered trademarks of REGENXBIO.
CONTACTS:
Dana Cormack
Corporate Communications
Dcormack@regenxbio.com
George E. MacDougall
Investor Relations
IR@regenxbio.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/regenxbio-highlights-key-2026-catalysts-and-announces-positive-long-term-functional-outcomes-in-lead-duchenne-gene-therapy-program-302657988.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/regenxbio-highlights-key-2026-catalysts-and-announces-positive-long-term-functional-outcomes-in-lead-duchenne-gene-therapy-program-302657988.html
SOURCE REGENXBIO Inc.