RenovoRx Announces Acceptance of Clinical Data Abstract at 2026 Society of Interventional Oncology Annual Scientific Meeting
Rhea-AI Summary
RenovoRx (Nasdaq: RNXT) announced acceptance of a clinical data abstract for presentation at the 2026 Society of Interventional Oncology Annual Scientific Meeting in Savannah, Feb 4–8, 2026.
The ePoster, presented Feb 6, 2026, examines real-time intravascular pressure measurements using the TAMP therapy platform and the FDA-cleared RenovoCath double-balloon catheter to optimize targeted chemotherapy delivery in solid tumors.
Positive
- None.
Negative
- None.
News Market Reaction
On the day this news was published, RNXT gained 0.20%, reflecting a mild positive market reaction. Argus tracked a peak move of +2.9% during that session. Our momentum scanner triggered 4 alerts that day, indicating moderate trading interest and price volatility. This price movement added approximately $71K to the company's valuation, bringing the market cap to $35M at that time.
Data tracked by StockTitan Argus on the day of publication.
Key Figures
Market Reality Check
Peers on Argus
RNXT fell 8.11% while peers showed mixed moves: PSTV -3.4%, BCAB -2.34%, RENB +19.33%, TELO 0%, TPST +1.73%. With no peers in the momentum scanner and no same-day peer news, the move appears stock-specific rather than a coordinated biotech sector reaction.
Previous Clinical trial Reports
| Date | Event | Sentiment | Move | Catalyst |
|---|---|---|---|---|
| Jan 08 | PK/PD sub-study data | Positive | +13.9% | Promising PK/PD data from TIGeR-PaC sub-study presented at ASCO GI 2026. |
| Dec 11 | Abstract acceptance | Positive | +1.6% | Acceptance of TIGeR-PaC PK/PD abstract at ASCO GI 2026 poster session. |
| Aug 14 | Interim DMC update | Positive | -22.9% | DMC recommended continuing pivotal Phase III TIGeR-PaC after interim review. |
| Apr 29 | Site enrollment | Neutral | -0.2% | Johns Hopkins joins and initiates enrollment in TIGeR-PaC clinical trial. |
| Apr 01 | Trial and revenue update | Positive | -3.0% | Initial RenovoCath revenues and TIGeR-PaC enrollment and cash position update. |
Clinical and trial-related updates often trigger sizable but inconsistent reactions, with several positive milestones met by negative price moves, indicating a history of divergence on some good news.
Over the past year, RNXT has repeatedly highlighted progress in its Phase III TIGeR-PaC program and related clinical milestones. Prior clinical-trial-tagged news included positive PK/PD data at ASCO GI 2026 with a +13.86% move, and multiple abstract acceptances showing reduced systemic drug levels for intra-arterial gemcitabine. Interim DMC review supported continuation of TIGeR-PaC, alongside growing RenovoCath® commercialization and cash balances. However, some of these constructive updates coincided with sharp declines, underscoring an uneven market response to clinical progress.
Historical Comparison
Clinical-trial-tagged news for RNXT has produced an average move of 8.34%, with prior abstract acceptances and TIGeR-PaC data updates often drawing meaningful but directionally mixed reactions.
Same-tag events show steady advancement of the Phase III TIGeR-PaC program, from site enrollment and interim DMC support to PK/PD sub-studies and multiple major conference abstracts, alongside early RenovoCath commercialization.
Regulatory & Risk Context
RNXT filed a Form S-3 shelf on 2025-11-14 to offer up to $50,000,000 of mixed securities, with at least one usage via a 424B5 prospectus supplement supporting an at-the-market program of up to $3,723,029. This provides a structured mechanism for future capital raises tied to TIGeR-PaC development and RenovoCath commercialization.
Market Pulse Summary
This announcement highlights acceptance of additional TAMP™ clinical data at the 2026 SIO meeting, extending prior TIGeR-PaC presentations at ASCO GI. Together, these updates underscore ongoing efforts to refine intra-arterial chemotherapy delivery via RenovoCath. Historically, RNXT has paired such clinical milestones with early commercialization progress and an active financing toolkit, including a $50,000,000 Form S-3 shelf, making both trial outcomes and funding choices important metrics to watch.
Key Terms
trans-arterial micro-perfusion medical
double balloon catheter medical
intravascular pressure medical
intra-arterial pressure medical
AI-generated analysis. Not financial advice.
Accepted Abstract Provides Supportive Evidence for the TAMP™ Therapy Platform’s Novel Approach to Targeted, Pressure-Mediated Drug-Delivery via Additional Clinical Data
MOUNTAIN VIEW, Calif., Jan. 28, 2026 (GLOBE NEWSWIRE) -- RenovoRx, Inc. (“RenovoRx” or “the Company”) (Nasdaq: RNXT), a life-sciences company developing innovative targeted oncology therapies and commercializing RenovoCath®, a patented, FDA-cleared drug-delivery device, today announced that its abstract submission to the 2026 Society of Interventional Oncology (SIO) Annual Scientific Meeting has been accepted. The meeting will be held February 4–8, 2026 in Savannah, Georgia.
Researchers including Christopher Laing, MD of Sutter Health in California, will present the clinical data abstract, “Real-Time Intravascular Pressure Measurements During Double Balloon Catheter-Mediated Trans-Arterial Micro-Perfusion to Optimize Drug Delivery in Solid Tumors.” The study explores a simplified method for measuring intra-arterial pressure during procedures that use the TAMP (Trans-Arterial Micro-Perfusion) therapy platform enabled by RenovoCath catheter, a double-balloon drug-delivery device, and how these measurements along with other variables can play a role in optimizing targeted delivery of chemotherapy in difficult-to-treat tumors.
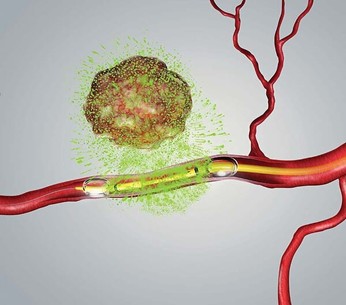
Illustration of RenovoCath delivering chemotherapy to tumor site via peripheral vascular system
“This translational study not only aims to validate a practical, real-time method for intra-arterial pressure monitoring during TAMP procedures using RenovoCath, but also may reinforce the potential for individualized, optimized drug-delivery with our proprietary technology,” said Dr. Ramtin Agah, RenovoRx’s Chairman and Chief Medical Officer and co-author of the study. “We are excited to present the full data with Dr. Laing at the SIO 2026 Meeting as we continue to demonstrate the potential benefits of our therapy platform to the marketplace.”
Abstract Details:
Presentation Date & Time: Friday, February 6, 2026, at 5:45-6:00 PM ET
Title: Real-Time Intravascular Pressure Measurements During Double Balloon Catheter-Mediated Trans-Arterial Micro Perfusion to Optimize Drug Delivery in Solid Tumors
Location: ePoster Corridor
Abstract Number: 303
Session Title: Science and Sips ePoster Reception
Authors: Christopher Laing, Robert Strasser, and Ramtin Agah
About RenovoCath
Based on its FDA clearance, RenovoCath® is intended for the isolation of blood flow and delivery of fluids, including diagnostic and/or therapeutic agents, to selected sites in the peripheral vascular system. RenovoCath is also indicated for temporary vessel occlusion in applications including arteriography, preoperative occlusion, and chemotherapeutic drug infusion. For further information regarding our RenovoCath Instructions for Use (“IFU”), please see: IFU-10004-Rev.-G-Universal-IFU.pdf.
About RenovoRx, Inc.
RenovoRx, Inc. (Nasdaq: RNXT) is a life sciences company developing innovative targeted oncology therapies and commercializing RenovoCath®, a novel, U.S. Food and Drug Administration (FDA)-cleared local drug-delivery device, targeting high unmet medical needs. RenovoRx’s patented Trans-Arterial Micro-Perfusion (TAMP™) therapy platform is designed for targeted therapeutic delivery across the arterial wall near the tumor site to bathe the target tumor, while potentially minimizing a therapy’s toxicities versus systemic intravenous therapy. RenovoRx’s novel approach to targeted treatment offers the potential for increased safety, tolerance, and improved efficacy, and its mission is to transform the lives of cancer patients by providing innovative solutions to enable targeted delivery of diagnostic and therapeutic agents.
RenovoRx is in the early stages of actively commercializing its TAMP technology and FDA-cleared RenovoCath as a stand-alone device. In December 2024, RenovoRx announced the receipt of its first commercial purchase orders for RenovoCath devices, and for the first nine months of 2025, approximately
RenovoRx is also evaluating its novel drug-device combination oncology product candidate (intra-arterial gemcitabine delivered via RenovoCath, known as IAG) in the ongoing Phase III TIGeR-PaC trial. IAG is being evaluated by the Center for Drug Evaluation and Research (the drug division of the FDA) under a U.S. investigational new drug application that is regulated by the FDA’s 21 CFR 312 pathway. IAG utilizes RenovoCath, the Company’s patented, FDA-cleared drug-delivery device, indicated for temporary vessel occlusion in applications including arteriography, preoperative occlusion, and chemotherapeutic drug infusion.
The combination product candidate (IAG), which is enabled by the RenovoCath device, is currently under investigation and has not been approved for commercial sale. RenovoCath with gemcitabine received Orphan Drug Designation for pancreatic cancer and bile duct cancer, which provides seven years of market exclusivity upon new drug application approval by the FDA.
For more information, visit www.renovorx.com. Follow RenovoRx on Facebook, LinkedIn, and X.
Cautionary Note Regarding Forward-Looking Statements
This press release and statements of the Company’s management made in connection therewith described herein contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, and Section 21E of the Securities Exchange Act of 1934, including but not limited to statements regarding (i) our clinical trials and studies, including the anticipated benefits to the Company of the clinical study abstract described herein, (ii) the potential for our product candidates to treat or provide clinically meaningful outcomes for certain medical conditions or diseases and (iii) our efforts to commercialize RenovoCath and our TAMP technology. Statements that are not purely historical are forward-looking statements. The forward-looking statements contained herein are based upon our current expectations and beliefs regarding future events, many of which, by their nature, are inherently uncertain, outside of our control and involve assumptions that may never materialize or may prove to be incorrect. These may include estimates, projections and statements relating to our research and development plans, intellectual property development, clinical trials, our therapy platform, business plans, financing plans, objectives and expected operating results, which are based on current expectations and assumptions that are subject to known and unknown risks and uncertainties that may cause actual results to differ materially and adversely from those expressed or implied by these forward-looking statements. These statements may be identified using words such as “may,” “expects,” “plans,” “aims,” “anticipates,” “believes,” “forecasts,” “estimates,” “intends,” and “potential,” or the negative of these terms or other comparable terminology regarding RenovoRx’s expectations strategy, plans or intentions, although not all forward-looking statements contain these words. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, that could cause actual events to differ materially from those projected or indicated by such statements, including, among other things: (i) the risk that our commercial sales efforts for RenovoCath and our TAMP technology may not lead to viable, revenue generating operations; (ii) circumstances which would adversely impact our ability to efficiently utilize our cash resources on hand or raise additional funding; (iii) the timing of the initiation, progress and potential results (including the results of interim analyses) of our preclinical studies, clinical trials and our research programs; (iv) the possibility that interim results may not be predictive of the outcome of our clinical trials, which may not demonstrate sufficient safety and efficacy to support regulatory approval of our product candidate; (v) that the applicable regulatory authorities may disagree with our interpretation of the data; research and clinical development plans and timelines, and the regulatory process for our product candidates; (vi) future potential regulatory milestones for our product candidates, including those related to current and planned clinical studies; (vii) our ability to use and expand our therapy platform to build a pipeline of product candidates; (viii) our ability to advance product candidates into, and successfully complete, clinical trials; (ix) the timing or likelihood of regulatory filings and approvals; (x) our estimates of the number of patients who suffer from the diseases we are targeting and the number of patients that may enroll in our clinical trials; (xi) the commercialization potential of our product candidates, if approved; (xii) our ability and the potential to successfully manufacture and supply our product candidates for clinical trials and for commercial use, if approved; (xiii) future strategic arrangements and/or collaborations and the potential benefits of such arrangements; (xiv) our estimates regarding expenses, future revenue, capital requirements and needs for additional financing and our ability to obtain additional capital; (xv) the sufficiency of our existing cash and cash equivalents to fund our future operating expenses and capital expenditure requirements; (xvi) our ability to retain the continued service of our key personnel and to identify, and hire and retain additional qualified personnel; (xvii) the implementation of our strategic plans for our business and product candidates; (xviii) the scope of protection we are able to establish and maintain for intellectual property rights, including our therapy platform, product candidates and research programs; (xix) our ability to contract with third-party suppliers and manufacturers and their ability to perform adequately; (xx) the pricing, coverage and reimbursement of our product candidates, if approved; and (xxi) developments relating to our competitors and our industry, including competing product candidates and therapies. Information regarding the foregoing and additional risks may be found in the section entitled “Risk Factors” in documents that we file from time to time with the Securities and Exchange Commission.
Forward-looking statements included herein are made as of the date hereof, and RenovoRx does not undertake any obligation to update publicly such forward-looking statements to reflect subsequent events or circumstances, except as required by law.
Contact:
KCSA Strategic Communications
Valter Pinto or Jack Perkins
T:212-896-1254
RenovoRx@KCSA.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/b55e00e6-3f1f-4454-901a-ffbfd3afc4be







