Tiziana Life Sciences Announces Additional Clinical Improvements Among Multiple Sclerosis Patients in its Expanded Access Program
70% of Patients in the Expanded Access Program (EAP) Have Seen Measurable Clinical Improvement in Their Fatigue After Six Months of Follow-up- All Patients Have Either Stabilized or Improved on Nasal Foralumab Treatment and No Patients Declined in Key Clinical Measures
NEW YORK, April 22, 2024 (GLOBE NEWSWIRE) -- Tiziana Life Sciences, Ltd. (Nasdaq: TLSA) (“Tiziana” or the “Company”), a biotechnology company developing breakthrough immunomodulation therapies via novel routes of drug delivery, today announced additional positive clinical results from its intermediate sized Expanded Access Program (EAP) for non-active secondary progressive multiple sclerosis (na-SPMS) patients. The data demonstrate multiple improvements in foralumab-treated patients, with
“All patients in this na-SPMS study had previously clinically progressed on ocrelizumab. They subsequently were enrolled in our EA program and received 6-months of intranasal foralumab,” stated Dr. Tanuja Chitnis, M.D., Principal Investigator and Professor of Neurology at Harvard Medical School and senior neurologist at Brigham and Women’s Hospital, a founding member of Mass General Brigham Healthcare System. “All 10 foralumab-treated patients stabilized or improved in key clinical measures, and seven showed clinical meaningful improvement in their fatigue at six months as measured by the MFIS. Other key clinical outcome measures included the Expanded Disability Status Scale (EDSS), Timed 25-Foot Walk Test (T25WT) and Pyramidal Scores in a disease state that typically shows a decline in function over time. I am pleased to see the continued clinical response to intranasal foralumab from patients enrolled in our expanded access na-SPMS program.”
Gabriele Cerrone, Chairman, acting CEO and founder of Tiziana Life Sciences, commented “Fatigue is a pervasive and challenging symptom for individuals living with MS, impacting their daily lives in profound ways. The clinically meaningful improvement in fatigue levels seen in seven out of ten patients, as well as the stabilization or improvements in other key clinical outcome measures that were seen in all patients, underscores the potential of Tiziana's investigational therapy to address this critical unmet need.”
Fatigue in MS, as measured via the MFIS, refers to an overwhelming sense of physical, mental, and emotional exhaustion that is disproportionate to the level of activity or effort exerted. It is a major, common, and often debilitating symptom experienced by many individuals with MS. It differs from the typical tiredness that everyone experiences from time to time. In the context of MS, it is called ‘primary fatigue’ and is a direct result of damage to the central nervous system. This kind of fatigue can significantly impact a person’s daily life and functioning.
The findings, which are summarized in Table 1 below, show broad-based six-month improvements across various key measures for multiple sclerosis. Secondary progressive multiple sclerosis is hallmarked by an increase of disability over time. The table below shows a stabilization or an improvement in physical function of the various clinical measures over a six-month period.
Table 1.
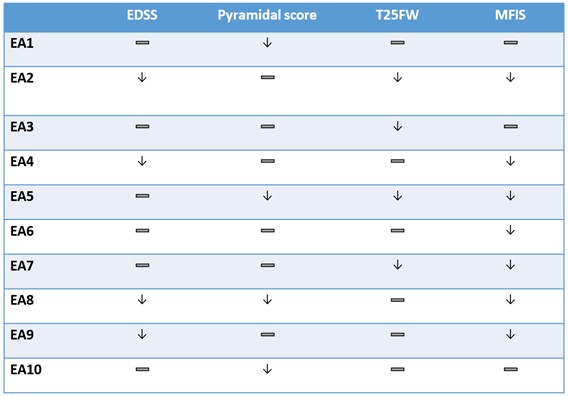
| — Denotes stabilization |
| ↓ Denotes improvement |
About Foralumab
Activated T cells play an important role in the inflammatory process. Foralumab, the only fully human anti-CD3 monoclonal antibody (mAb), binds to the T cell receptor and dampens inflammation by modulating T cell function, thereby suppressing effector features in multiple immune cell subsets. This effect has been demonstrated in patients with COVID and with multiple sclerosis, as well as in healthy normal subjects. The non-active SPMS intranasal foralumab Phase 2 trial began screening patients in November of 2023. Immunomodulation by nasal anti-CD3 mAb represents a novel avenue for treatment of neuroinflammatory and neurodegenerative human diseases.[1],[2]
About Tiziana Life Sciences
Tiziana Life Sciences is a clinical-stage biopharmaceutical company developing breakthrough therapies using transformational drug delivery technologies to enable alternative routes of immunotherapy. Tiziana’s innovative nasal approach has the potential to provide an improvement in efficacy as well as safety and tolerability compared to intravenous (IV) delivery. Tiziana’s lead candidate, intranasal foralumab, which is the only fully human anti-CD3 mAb, has demonstrated a favorable safety profile and clinical response in patients in studies to date. Tiziana’s technology for alternative routes of immunotherapy has been patented with several applications pending and is expected to allow for broad pipeline applications.
Forward-Looking Statements
Certain statements made in this announcement are forward-looking statements. These forward-looking statements are not historical facts but rather are based on the Company's current expectations, estimates, and projections about its industry, its beliefs, and assumptions. Words such as 'anticipates,' 'expects,' 'intends,' 'plans,' 'believes,' 'seeks,' 'estimates,' and similar expressions are intended to identify forward-looking statements. These statements are not guarantees of future performance and are subject to known and unknown risks, uncertainties, and other factors, some of which are beyond the Company's control, are difficult to predict, and could cause actual results to differ materially from those expressed or forecasted in the forward-looking statements. The Company cautions security holders and prospective security holders not to place undue reliance on these forward-looking statements, which reflect the view of the Company only as of the date of this announcement. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including: the uncertainties related to market conditions and other factors described more fully in the section entitled ‘Risk Factors’ in Tiziana’s Annual Report on Form 20-F for the year ended December 31, 2022, and other periodic reports filed with the Securities and Exchange Commission. The forward-looking statements made in this announcement relate only to events as of the date on which the statements are made. The Company will not undertake any obligation to release publicly any revisions or updates to these forward-looking statements to reflect events, circumstances, or unanticipated events occurring after the date of this announcement except as required by law or by any appropriate regulatory authority.
For further inquiries:
Tiziana Life Sciences Ltd
Paul Spencer, Business Development and Investor Relations
+44 (0) 207 495 2379
email: info@tizianalifesciences.com
Investors:
Irina Koffler
LifeSci Advisors, LLC
646.970.4681
ikoffler@lifesciadvisors.com
[1] https://www.pnas.org/doi/10.1073/pnas.2220272120
[2] https://www.pnas.org/doi/10.1073/pnas.2309221120
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/bbf0c43f-9388-4ac1-a37c-b09769dafeef








