Tempest Announces Closing of Strategic Acquisition of Dual-Targeting CAR-T Assets
Rhea-AI Summary
Tempest (Nasdaq: TPST) closed an all-stock acquisition of dual-targeting CAR-T programs and secured strategic partner funding from Factor Bioscience, adding clinical-stage assets including TPST-2003. The deal extends operational runway to mid-2027, supports multiple near-term milestones, and installs Matt Angel, Ph.D., as President and CEO.
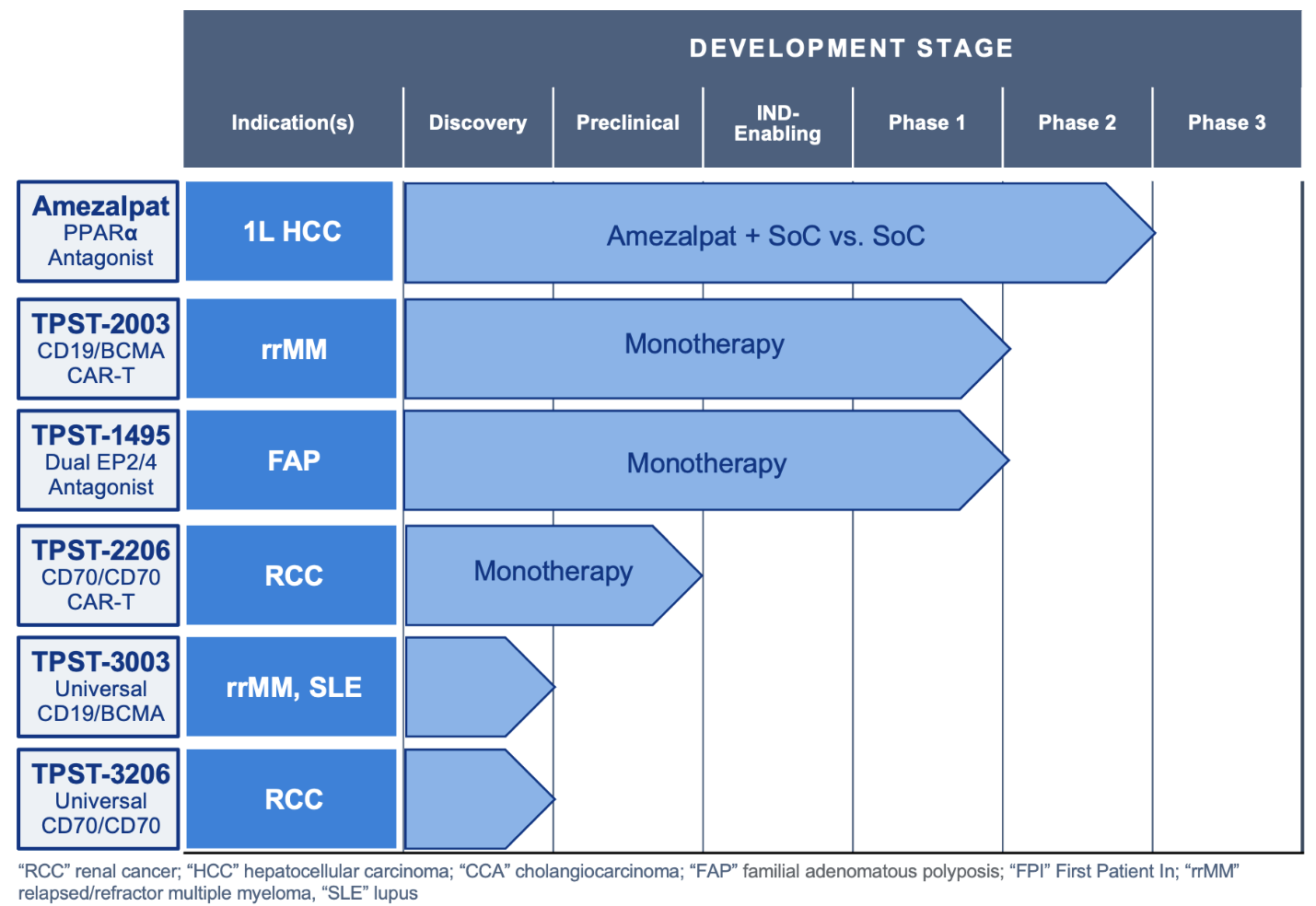
Key program highlights: TPST-2003 has completed a Phase 1 rrMM study with China BLA planned for 2027; TPST-2003 is enrolling POEMS patients with data and a China BLA planned by 2028; TPST-1120 remains Phase 3 ready in first-line HCC.
Positive
- Adds clinical-stage CAR-T asset TPST-2003 with Phase 1 complete
- Strategic partner funds China development, reducing Tempest cash burden
- Operational runway extended to mid-2027
- TPST-1120 (amezalpat) is Phase 3 ready in first-line HCC
Negative
- All-stock consideration and share issuance to Factor may dilute shareholders
- Runway limited to mid-2027, creating near-term financing/de-risking pressure
- China development funded by partner creates dependence on third-party execution
News Market Reaction – TPST
On the day this news was published, TPST declined 1.24%, reflecting a mild negative market reaction. Argus tracked a peak move of +16.1% during that session. Argus tracked a trough of -32.6% from its starting point during tracking. Our momentum scanner triggered 18 alerts that day, indicating notable trading interest and price volatility. This price movement removed approximately $147K from the company's valuation, bringing the market cap to $12M at that time. Trading volume was exceptionally heavy at 7.1x the daily average, suggesting significant selling pressure.
Data tracked by StockTitan Argus on the day of publication.
Key Figures
Market Reality Check
Peers on Argus
Sector peers show mixed moves, with names like BRNS down 3.11% and ACRV down 3.16%, while PSTV and RADX are modestly positive, pointing to stock-specific rather than broad biotech pressure for TPST.
Previous Acquisition Reports
| Date | Event | Sentiment | Move | Catalyst |
|---|---|---|---|---|
| Nov 19 | Strategic acquisition | Positive | -48.6% | Announced all-stock acquisition of Factor dual-CAR T programs and runway extension. |
The only prior acquisition headline over the last six months coincided with a sharp -48.59% one-day decline, indicating past acquisition news was met with significant selling.
Over the past six months, Tempest has repeatedly used strategic transactions and financings to reshape its outlook. On Nov 19, 2025, it announced an all‑stock acquisition of Factor’s dual‑CAR T programs, issuing 8,268,495 shares (about 65% of outstanding) and securing an investment commitment expected to extend its runway to mid 2027. That announcement triggered a -48.59% price move, showing that equity-heavy deal structures have previously been met with substantial pressure, context that frames today’s closing of the same transaction.
Historical Comparison
In the past 12 months, Tempest had one prior acquisition headline, which saw a -48.59% move. That suggests acquisition-related news has historically coincided with pronounced downside.
This closing announcement finalizes the Nov 2025 all‑stock acquisition of Factor’s dual‑CAR T programs, maintaining the projected cash runway to mid 2027 and transitioning from agreement to integrated CAR‑T pipeline execution.
Market Pulse Summary
This announcement closes a previously agreed all‑stock acquisition, giving Tempest a broader CAR‑T pipeline and extending its operational runway to mid-2027. Key assets include TPST‑2003, with completed Phase 1 work in rrMM and China BLA plans in 2027–2028, plus upcoming TPST‑1495 Phase 2 work funded by the NCI. Historically, acquisition news in Nov 2025 coincided with a -48.59% move, so investors may focus on dilution history, execution on new trials, and milestone delivery against stated timelines.
Key Terms
car-t medical
chimeric antigen receptor medical
multiple myeloma medical
poems syndrome medical
familial adenomatous polyposis medical
renal cell carcinoma medical
allogeneic medical
AI-generated analysis. Not financial advice.
- All-stock transaction brings Tempest a portfolio of next-generation CAR-T assets, including TPST-2003, a clinical-stage dual-targeting CD-19/BCMA CAR-T with strategic partner-funded BLA filing in China planned for 2027
- Operational runway extended to mid-2027, supporting multiple potential value-generating milestones
- Matt Angel, Ph.D., joins Tempest as President and CEO
- Previously announced transaction closed following stockholder approval of share issuance at the 2025 Annual Meeting on January 27, 2026
BRISBANE, Calif., Feb. 04, 2026 (GLOBE NEWSWIRE) -- Tempest Therapeutics, Inc. (Nasdaq: TPST) (“Tempest”), a clinical-stage biotechnology company with a pipeline of advanced strategic therapeutic assets, today announced the closing of a previously announced transaction pursuant to which Tempest acquired certain dual-targeting chimeric antigen receptor (CAR)-T programs and obtained financing support from Factor Bioscience Inc. and its affiliates (collectively, “Factor”) in an all-stock transaction resulting in a diverse portfolio including clinical-stage product candidates and an extended runway with multiple potential near-term milestones (the “Transaction”).
“I am excited to join the Tempest team and to have the opportunity to develop this innovative pipeline of potential therapies to treat a range of solid tumors and hematologic malignancies,” said Dr. Matt Angel, President and Chief Executive Officer of Tempest. “I look forward to advancing the company’s vision of bringing important therapies to patients.”
“The Board is pleased to announce the closing of this transaction, which not only provides increased financial stability for Tempest, but also the opportunity for potentially significant milestones over the next 12-18 months from both the legacy small molecule programs and the new cell therapy assets,” said Stephen Brady, Chair of the Board.
“As Tempest moves into this next phase, we would like to thank Geoff Nichol and Mike Raab.” Mr. Brady continued. “We are grateful for Geoff’s support, engagement and inquisitive mind since he joined the Board in 2021, and wish him continued success in his endeavors. Mike Raab has been our Chair since 2018, during which time he provided clear leadership, thoughtful perspectives, and significant contributions to Tempest throughout his tenure. We are thrilled that Mike will continue to serve on the Board, bringing the ongoing benefit of his experience and guidance to the company.”
Key Takeaways:
- Amezalpat (TPST-1120) remains Phase 3 ready in first-line liver cancer (“HCC”), supported by global regulatory agreement and positive randomized Phase 2 data. Tempest plans to pursue business development discussions to advance pivotal development.
- TPST-2003: new dual-targeting CD19/BCMA CAR-T asset
- Phase 1 complete in patients with relapsed/refractory multiple myeloma (“rrMM”), with data expected in 2026 and a biologics license application (“BLA”) in China planned for 2027
- Phase 1 currently enrolling patients with POEMS syndrome, with data expected in 2027 and a BLA in China planned for 2028
- Tempest has global rights to TPST-2003 outside of China, India, Turkey and Russia, and plans to pursue a potential registrational study in rrMM in the U.S. starting in 2027
- Pivotal data from the Chinese study expected to validate probability of success for the program, and rights include the right to reference data generated in support of the planned China BLA
- All development activities in China to be funded by strategic partner
- Tempest expects a Phase 2 study of TPST-1495 in familial adenomatous polyposis (“FAP”) to enroll the first patient in Q1’26 and to be funded by the National Cancer Institute and operationalized by the Cancer Prevention Clinical Trials Network.
- Plan to continue the development of additional new preclinical and research-stage pipeline programs:
- TPST-2206: dual-targeting CD70/CD70 CAR-T for renal cell carcinoma
- TPST-3003: allogeneic dual-targeting CD19/BCMA
- TPST-3206: allogeneic dual-targeting CD70/CD70
- Existing cash and an investment commitment from Factor is expected to provide a runway to mid-2027 and potentially through key data milestones.
Combined Pipeline
Advisors
MTS Health Partners, L.P. served as financial advisor to Tempest, and Cooley LLP served as legal advisor. In addition, MTS Securities, LLC (an affiliate of MTS Health Partners, L.P.) provided an opinion to the board of directors of Tempest regarding the fairness of the purchase price to be paid by Tempest to Factor in connection with the Transaction, subject to the qualifications and limitations set forth therein. Morse, Barnes-Brown & Pendleton, P.C. served as legal advisor to Factor.
About Tempest Therapeutics
Tempest Therapeutics is a clinical-stage biotechnology company with a diverse portfolio of cell therapy and small molecule product candidates that leverage tumor-targeted and/or immune-mediated mechanisms to potentially treat a wide range of cancers. Tempest is headquartered in Brisbane, California. More information about Tempest can be found on the company’s website at https://www.tempesttx.com.
Forward-Looking Statements
This press release contains forward-looking statements (including within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended (the “Securities Act”)) concerning Tempest Therapeutics, Inc. These statements may discuss goals, intentions, and expectations as to future plans, trends, events, results of operations or financial condition, or otherwise, based on current beliefs of the management of Tempest Therapeutics, as well as assumptions made by, and information currently available to, management of Tempest Therapeutics. Forward-looking statements contained in this press release include but are not limited to statements relating to: the investment commitment and extension of Tempest Therapeutics’ cash runway through mid-2027; the potential benefits of the Transaction, including the combination of the programs and ability to benefit patients; the design, initiation, progress, timing, scope and results of clinical trials, including the anticipated Phase 3 study for amezalpat and the advancement of TPST-2003; the expectation that the pivotal data from the Chinese study will validate the probability of success for TPST-2003; the anticipated China BLA; the expected Phase 2 study for TPST-1405 and the timing for first patient enrollment and the funding therefor; the planned continued development of additional new preclinical and research-stage pipeline programs; anticipated therapeutic benefit and regulatory development of Tempest Therapeutics’ product candidates; and Tempest Therapeutics’ ability to achieve its operational plans. Any forward-looking statements in this press release are based on Tempest Therapeutics’ current expectations, estimates and projections about its industry as well as management’s current beliefs and expectations of future events only as of today and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements. These risks and uncertainties include, but are not limited to Tempest Therapeutics’ need for additional capital to fund its planned programs and operations and to continue to operate as a going concern; unexpected safety or efficacy data observed during preclinical or clinical trials; funding from the National Cancer Institute for the expected Phase 2 study of TPST-1405 may not be available at the levels anticipated, or at all, in which case Tempest Therapeutics’ decision and ability to move forward with this trial will be subject to holistic program considerations and capital availability; past results may not be indicative of future results; clinical trial site activation or enrollment rates that are lower than expected; loss of key personnel; changes in expected or existing competition; changes in the regulatory environment; risks relating to volatility and uncertainty in the capital markets for biotechnology companies; and unexpected litigation or other disputes. These and other factors that may cause actual results to differ from those expressed or implied are discussed in greater detail in the “Risk Factors” section of Tempest’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2025, the “Risk Factors” section under Proposal 5 contained in Tempest’s definitive proxy statement on Schedule 14A, filed with the Securities and Exchange Commission (“SEC”) on December 31, 2025, and in other documents filed by Tempest from time to time with the SEC. Except as required by applicable law, Tempest Therapeutics undertakes no obligation to revise or update any forward-looking statement, or to make any other forward-looking statements, whether as a result of new information, future events or otherwise. These forward-looking statements should not be relied upon as representing Tempest Therapeutics’ views as of any date subsequent to the date of this press release and should not be relied upon as prediction of future events. In light of the foregoing, investors are urged not to rely on any forward-looking statement in reaching any conclusion or making any investment decision about any securities of Tempest Therapeutics.
Investor Contacts:
Sylvia Wheeler
Wheelhouse Life Science Advisors
swheeler@wheelhouselsa.com
Aljanae Reynolds
Wheelhouse Life Science Advisors
areynolds@wheelhouselsa.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/246ed768-1ec8-4ca2-9d36-d3ca777afad9








