The Evolution of Fourth - Fifth Generation HIV Rapid Test Kits is Booming, the Market to Create USD 2 Billion Opportunities by 2028 - Arizton
The global HIV rapid test kits market is projected to grow from
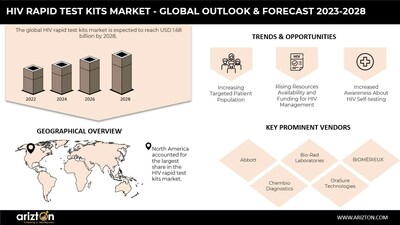
- Projected market growth from USD 1.43 billion in 2022 to USD 1.68 billion by 2028.
- Increased adoption of fourth- and fifth-generation HIV rapid test kits, enhancing reliability.
- Growth driven by government initiatives and digital innovations in HIV self-testing.
- High traction of digital health technologies in healthcare services.
- Potential challenges in addressing false negatives reported from some tests.
- Concerns over the accessibility and awareness of HIV self-testing tools.
Insights
Analyzing...
The application of fourth-generation and fifth-generation rapid test kits is currently high. The adoption and innovation of digital HIV self-testing tools have increased in recent times, with website-based innovation becoming highly popular.
Moreover, the introduction of digital support for HIV self-testing and government initiatives to boost the sales of the market. The application of digital tools in HIV management is increasing rapidly, which is expected to fuel the adoption of HIV self-testing rapid test kits. These interventions are currently improving HIV care and diagnosis. In 2021–2022, the HIV Self-Testing (HIVST) deployment and digital tool integrations in health services were emerging, but it was a priority for UNAIDS. In healthcare services, digital health technologies are achieving high traction in high-and middle-income countries. Furthermore, telemedicine has allowed the continuity of HIV care for many patients. The COVID-19 pandemic covers the roadmap for the evolution of HIV self-testing with digital support to reduce the gap in HIV care deliveries. . The application of digital tools in HIV management is increasing rapidly, which is expected to fuel the adoption of HIV self-testing rapid test kits. These interventions are currently improving HIV care and diagnosis. In 2021–2022, the HIV Self-Testing (HIVST) deployment and digital tool integrations in health services were at an emerging stage, but it was a priority for UNAIDS. In healthcare services, digital health technologies are achieving high traction in high-and middle-income countries. Furthermore, telemedicine has allowed the continuity of HIV care for many patients. The COVID-19 pandemic covers the roadmap for the evolution of HIV self-testing with digital support to reduce the gap in HIV care deliveries.
Implementing digital tools in the HIV rapid test kits market has a high potential to reach diverse audiences with the potential for widespread scale-up. The effective and efficient linkage between health services and patients increasing access to HIV testing. These factors are expected to increase HIV rapid test adoption in the diverse patient population, thereby driving the demand for market growth in upcoming years. According to the
Global HIV Rapid Test Kits Market Report Scope
Report Attributes | Details |
Market Size (2028) | |
Market Size (2022) | |
CAGR (2022-2028) | 2.70 % |
Base Year | 2022 |
Forecast Year | 2023-2028 |
Market Segments | Technology, Sample Type, Distribution Channels, and Geography |
Geographic Analysis | |
Countries Covered | The US, |
Key Leading Vendors | |
Market Dynamics |
|
Customization Request | If our report does not include the information you are searching for, you may contact us to have a report tailored to your specific business needs https://www.arizton.com/customize-report/3697 |
Click Here to Download the Free Sample Report
According to the
ADOPTION OF INNOVATION LATERAL FLOW IMMUNOASSAY IS HIGH ACROSS HIV RAPID TEST KITS MARKET
Several companies in the market are coming up with low-cost lateral flow-based HIV test kits. Many regulatory bodies, such as the WHO and other NGOs, are funding HIV test kit manufacturing. This will help companies to come up with unique products in the market. The level of innovations in the lateral flow immune assay is high in the market. In LMICs, there is a constant rise in the prevalence of HIV infection, which demands the usage of HIV rapid diagnostic test kits to increase significantly. Lateral flow immune assays are more user-friendly and are highly used among people with limited access to healthcare settings. Most health authorities and healthcare settings suggest immunochromatographic rapid test kits. In western African countries, the newly developed and available HIV rapid test kits, which are developed based on the immunochromatographic assay, are commonly used. Market players expect to have high market growth opportunities in
Click Here to Download the Free Sample Report
KEY COMPANY PROFILES
Abbott - Bio-Rad Laboratories
- BIOMÉRIEUX
- Chembio Diagnostics
- OraSure Technologies
- Atomo Diagnostics
- AccuBioTech
- bioLytical Laboratories
- Biosynex
- Cupid Limited
- DIALAB
- HUMAN
- INTEC
J. Mitra & Co- Meril Life Sciences
MP Biomedicals - Medsource Ozone Biomedicals
- Nanjing Synthgene Medical Technology
- Premier Medical
- SD Biosensor
- KHB (Shanghai Kehua Bio-Engineering co., Ltd.)
- Türklab
A.S . Trinity Biotech - Wantai BioPharma
- Wondfo
MARKET SEGMENTATION
Technology
- Lateral Flow Immunoassay
- Immunofiltration
Sample Type
- Blood
- Oral Fluid
- Urine
Distribution Channels
- Offline
- Online
Geography
North America - US
Canada Europe France Italy Ukraine Portugal Russia - APAC
China India Indonesia Thailand Vietnam Latin America Brazil Mexico Columbia Middle East &Africa South Africa Mozambique Kenya Nigeria Tanzania
CHECK OUT SOME OF THE TOP-SELLING RESEARCH RELATED REPORTS
Fertility Test Market - Global Outlook & Forecast 2022-2027: The global fertility test market is expected to reach
Lateral Flow Assays Market - Global Outlook & Forecast 2023-2028: The global lateral flow assays market is expected to reach
COVID-19 Rapid Antigen Test Kits Market - Global Outlook & Forecast 2022-2027: The COVID-19 rapid antigen tests market is expected to reach
DNA Extraction
WHY ARIZTON?
24x7 availability – we are always there when you need us
200+ Fortune 500 Companies trust Arizton's report
5+ years of experience in delivering Insights at the Global, Regional & Country Level
TABLE OF CONTENT
1 RESEARCH METHODOLOGY
2 RESEARCH OBJECTIVES
3 RESEARCH PROCESS
4
4.1 MARKET DEFINITION
4.1.1 INCLUSIONS
4.1.2 EXCLUSIONS
4.1.3 MARKET ESTIMATION CAVEATS
4.2 BASE YEAR
4.3 SCOPE OF THE STUDY
4.3.1 MARKET BY TECHNOLOGY
4.3.2 MARKET BY SAMPLE TYPE
4.3.3 MARKET BY DISTRIBUTION CHANNELS
4.3.4 MARKET SEGMENTATION BY GEOGRAPHY
5 REPORT ASSUMPTIONS & CAVEATS
5.1 KEY CAVEATS
5.2 CURRENCY CONVERSION
5.3 MARKET DERIVATION
6 MARKET AT A GLANCE
7 PREMIUM INSIGHTS
7.1 OVERVIEW
8 INTRODUCTION
8.1 OVERVIEW
9 MARKET OPPORTUNITIES & TRENDS
9.1 INTRODUCTION OF NEXT GENERATION HIV RAPID TEST KITS
9.2 INCREASE IN HIV SELF-TESTING BY GOVERNMENT BODIES
9.3 DIGITAL SUPPORT FOR HIV SELF-TESTING
10 MARKET GROWTH ENABLERS
10.1 INCREASING HIV PATIENT POPULATION
10.2 INCREASED RESOURCES AVAILABILITY AND FUNDING FOR HIV MANAGEMENT
10.3 INCREASED AWARENESS OF HIV SELF-TESTING
11 MARKET RESTRAINTS
11.1 INCREASING INCIDENCES OF FALSE NEGATIVES
11.2 LIMITATIONS & CHALLENGES ASSOCIATED WITH HIV TESTING
11.3 LOW ACCESSIBLITY & LACK OF AWARENESS FOR HIVST
12 MARKET LANDSCAPE
12.1 MARKET OVERVIEW
12.1.1 INSIGHTS BY TECHNOLOGY
12.1.2 INSIGHTS BY SAMPLE TYPE
12.1.3 INSIGHTS BY DISTRIBUTION CHANNELS
12.1.4 INSIGHTS BY GEOGRAPHY
12.2 MARKET SIZE & FORECAST
12.3 FIVE FORCES ANALYSIS
12.3.1 THREAT OF NEW ENTRANTS
12.3.2 BARGAINING POWER OF SUPPLIERS
12.3.3 BARGAINING POWER OF BUYERS
12.3.4 THREAT OF SUBSTITUTES
12.3.5 COMPETITIVE RIVALRY
13 TECHNOLOGY
13.1 MARKET SNAPSHOT & GROWTH ENGINE
13.2 MARKET OVERVIEW
13.3 LATERAL FLOW IMMUNOASSAY
13.3.1 MARKET OVERVIEW
13.3.2 MARKET SIZE & FORECAST
13.3.3 MARKET BY GEOGRAPHY
13.4 IMMUNOFILTRATION
13.4.1 MARKET OVERVIEW
13.4.2 MARKET SIZE & FORECAST
13.4.3 MARKET BY GEOGRAPHY
14 SAMPLE TYPE
14.1 MARKET SNAPSHOT & GROWTH ENGINE
14.2 MARKET OVERVIEW
14.3 BLOOD
14.3.1 MARKET OVERVIEW
14.3.2 MARKET SIZE & FORECAST
14.3.3 MARKET BY GEOGRAPHY
14.4 ORAL FLUID
14.4.1 MARKET OVERVIEW
14.4.2 MARKET SIZE & FORECAST
14.4.3 MARKET BY GEOGRAPHY
14.5 URINE
14.5.1 MARKET OVERVIEW
14.5.2 MARKET SIZE & FORECAST
14.5.3 MARKET BY GEOGRAPHY
15 DISTRIBUTION CHANNEL
15.1 MARKET SNAPSHOT & GROWTH ENGINE
15.2 MARKET OVERVIEW
15.3 OFFLINE
15.3.1 MARKET OVERVIEW
15.3.2 MARKET SIZE & FORECAST
15.3.3 MARKET BY GEOGRAPHY
15.4 ONLINE
15.4.1 MARKET OVERVIEW
15.4.2 MARKET SIZE & FORECAST
15.4.3 MARKET BY GEOGRAPHY
16 GEOGRAPHY
16.1 MARKET SNAPSHOT & GROWTH ENGINE
16.2 GEOGRAPHIC OVERVIEW
17 NORTH AMERICA
17.1 MARKET OVERVIEW
17.2 MARKET SIZE & FORECAST
17.3 TECHNOLOGY
17.3.1 MARKET SIZE & FORECAST
17.4 SAMPLE TYPE
17.4.1 MARKET SIZE & FORECAST
17.5 DISTRIBUTION CHANNELS
17.5.1 MARKET SIZE & FORECAST
17.6 KEY COUNTRIES
17.6.1 US: MARKET SIZE & FORECAST
17.6.2
18 APAC
18.1 MARKET OVERVIEW
18.2 MARKET SIZE & FORECAST
18.3 TECHNOLOGY
18.3.1 MARKET SIZE & FORECAST
18.4 SAMPLE TYPE
18.4.1 MARKET SIZE & FORECAST
18.5 DISTRIBUTION CHANNELS
18.5.1 MARKET SIZE & FORECAST
18.6 KEY COUNTRIES
18.6.1
18.6.2
18.6.3
18.6.4
18.6.5
19 MIDDLE EAST &
19.1 MARKET OVERVIEW
19.2 MARKET SIZE & FORECAST
19.3 TECHNOLOGY
19.3.1 MARKET SIZE & FORECAST
19.4 SAMPLE TYPE
19.4.1 MARKET SIZE & FORECAST
19.5 DISTRIBUTION CHANNELS
19.5.1 MARKET SIZE & FORECAST
19.6 KEY COUNTRIES
19.6.1
19.6.2
19.6.3
19.6.4
19.6.5
20 EUROPE
20.1 MARKET OVERVIEW
20.2 MARKET SIZE & FORECAST
20.3 TECHNOLOGY
20.3.1 MARKET SIZE & FORECAST
20.4 SAMPLE TYPE
20.4.1 MARKET SIZE & FORECAST
20.5 DISTRIBUTION CHANNELS
20.5.1 MARKET SIZE & FORECAST
20.6 KEY COUNTRIES
20.6.1
20.6.2
20.6.3
20.6.4
20.6.5
21 LATIN AMERICA
21.1 MARKET OVERVIEW
21.2 MARKET SIZE & FORECAST
21.3 TECHNOLOGY
21.3.1 MARKET SIZE & FORECAST
21.4 SAMPLE TYPE
21.4.1 MARKET SIZE & FORECAST
21.5 DISTRIBUTION CHANNELS
21.5.1 MARKET SIZE & FORECAST
21.6 KEY COUNTRIES
21.6.1
21.6.2
21.6.3
22 COMPETITIVE LANDSCAPE
22.1 COMPETITION OVERVIEW
22.2 MARKET SHARE ANALYSIS
22.2.1
22.2.2 BIO-RAD LABORATORIES
22.2.3 BIOMÉRIEUX
22.2.4 CHEMBIO DIAGNOSTICS
22.2.5 ORASURE TECHNOLOGIES
23 KEY COMPANY PROFILES
23.1
23.1.1 BUSINESS OVERVIEW
23.1.2 PRODUCT OFFERINGS
23.1.3 KEY STRATEGIES
23.1.4 KEY STRENGTHS
23.1.5 KEY OPPORTUNITIES
23.2 BIO-RAD LABORATORIES
23.3 BIOMÉRIEUX
23.4 CHEMBIO DIAGNOSTICS
23.5 ORASURE TECHNOLOGIES
24 OTHER PROMINENT VENDORS
24.1 ATOMO DIAGNOSTICS
24.1.1 BUSINESS OVERVIEW
24.1.2 PRODUCT OFFERINGS
24.2 ACCUBIOTECH
24.3 BIOLYTICAL LABORATORIES
24.4 BIOSYNEX
24.5 CUPID LIMITED
24.6 HUMAN
24.7 INTEC
24.8
24.9 MERIL LIFE SCIENCES
24.10
24.11 MEDSOURCE OZONE BIOMEDICALS
24.12
24.13 PREMIER MEDICAL
24.14 SD BIOSENSOR
24.15 KHB (SHANGHAI KEHUA BIO-ENGINEERING)
24.16 TÜRKLAB A.S.
24.17
24.18 WANTAI BIOPHARMA
24.19 WONDFO
25 REPORT SUMMARY
25.1 KEY TAKEAWAYS
25.2 STRATEGIC RECOMMENDATIONS
26 QUANTITATIVE SUMMARY
26.1 MARKET BY GEOGRAPHY
26.2 MARKET BY TECHNOLOGY
26.3 MARKET BY SAMPLE TYPE
26.4 MARKET BY DISTRIBUTION CHANNELS
26.5 TECHNOLOGY: MARKET BY GEOGRAPHY
26.5.1 LATERAL FLOW IMMUNOASSAY: MARKET BY GEOGRAPHY
26.5.2 IMMUNOFILTRATION: MARKET BY GEOGRAPHY
26.6 SAMPLE TYPE: MARKET BY GEOGRAPHY
26.6.1 BLOOD SAMPLE TYPE: MARKET BY GEOGRAPHY
26.6.2 ORAL FLUID SAMPLE TYPE: MARKET BY GEOGRAPHY
26.6.3 URINE SAMPLE TYPE: MARKET BY GEOGRAPHY
26.7 DISTRIBUTION CHANNELS: MARKET BY GEOGRAPHY
26.7.1 OFFLINE DISTRIBUTION CHANNELS: MARKET BY GEOGRAPHY
26.7.2 ONLINE DISTRIBUTION CHANNELS: MARKET BY GEOGRAPHY
27 APPENDIX
27.1 ABBREVIATIONS
About Us:
Arizton Advisory and Intelligence is an innovative and quality-driven firm that offers cutting-edge research solutions to clients worldwide. We excel in providing comprehensive market intelligence reports and advisory and consulting services.
We offer comprehensive market research reports on consumer goods & retail technology, automotive and mobility, smart tech, healthcare, life sciences, industrial machinery, chemicals, materials, I.T. and media, logistics, and packaging. These reports contain detailed industry analysis, market size, share, growth drivers, and trend forecasts.
Arizton comprises a team of exuberant and well-experienced analysts who have mastered generating incisive reports. Our specialist analysts possess exemplary skills in market research. We train our team in advanced research practices, techniques, and ethics to outperform in fabricating impregnable research reports.
Call: +1-312-235-2040
+1 302 469 0707
Mail: enquiry@arizton.com
Photo: https://mma.prnewswire.com/media/1984906/HIV_Rapid_Tests_Kit_Market.jpg
Logo: https://mma.prnewswire.com/media/818553/Arizton_Logo.jpg
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/the-evolution-of-fourth--fifth-generation-hiv-rapid-test-kits-is-booming-the-market-to-create-usd-2-billion-opportunities-by-2028---arizton-301723724.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/the-evolution-of-fourth--fifth-generation-hiv-rapid-test-kits-is-booming-the-market-to-create-usd-2-billion-opportunities-by-2028---arizton-301723724.html
SOURCE Arizton Advisory & Intelligence