UCB on Growth Path for a Decade Plus
- Revenue in 2023 reached
€ 5.25 billion (-5% ; -6% CER1), net sales were€ 4.87 billion (-5% ; -6% CER1) – in-line with financial guidance - Strong performance by newly launched growth drivers (net sales growth at Act rates): EVENITY® +
140% , FINTEPLA® +94% , BIMZELX® +323% . RYSTIGGO® with€ 19 million since July, ZILBRYSQ® launched globally since Q1 2024 - Underlying profitability (adj. EBITDA2) was
€ 1.35 billion (+7% ; -1% CER1),25.7% of revenue – better than the guidance due to higher EVENITY® contribution and good cost management U.S. FDA accepted the filings of BIMZELX® for psoriatic arthritis (PsA), non-radiographic axial spondyloarthritis (nr-axSpA) and ankylosing spondylitis (AS). The application for hidradenitis suppurativa (HS) has also been submitted to FDA. FDA action and potential approvals expected for all indications before the end of 2024- Financial guidance for 2024: Revenue expected to grow to
€ 5.5 -5.7 billion, adjusted EBITDA2 23.0-24.5% of revenue, Core EPS3 of€ 3.70 -4.40
"Our 2023 performance showcases our unwavering commitment to ensuring people with severe diseases can live the life they like, as free as possible from challenges of disease, reaching more than 3.2 million patients globally with severe immunological and neurological conditions. In the last 14 months we obtained 14 approvals, across 6 patient populations and across 3 continents, fuelling our growth for a decade plus. As an example, superior patient experience and UCB's dedication have allowed to double the number of patients using BIMZELX ® in
FY 2023 revenue reached
Underlying profitability (adjusted EBITDA2) reached
Profit amounted to
Sandrine Dufour, CFO UCB says: "A year with good product growth and strong launches - we are pleased to deliver again solid financial results. As expected, we're seeing the impacts from the losses of exclusivity for two products diminishing in the second half and thanks to the strong revenue performance of our growth assets, we returned to growth in the second half with almost +
Regulatory and Clinical Pipeline Update
UCB continuously innovates and strives to find new ways to deliver solutions to people living with severe immunological and neurological diseases, leading in 2023 to a clinical development pipeline with 12 clinical programs ongoing spanning 10 different medicines, set to help 10 different patient populations. Since January 2023 and in the key regions
Regulatory Update
In June 2023, E KEPPRA® (levetiracetam) was approved in
In July 2023, the European Medicines Agency (EMA) has accepted for review the marketing authorization application of bimekizumab for the treatment of adults with moderate to severe hidradenitis suppurativa (HS), a chronic, recurrent, and debilitating skin condition with high unmet medical need.
In July 2023, UCB submitted the marketing authorization application for the epilepsy medicine BRIVIACT® (brivaracetam) to PMDA in
In September 2023, UCB announced the approval of RYSTIGGO® (rozanolixizumab) and ZILBRYSQ® (zilucoplan) for the treatment of adult patients with generalized myasthenia gravis (gMG) in
In October 2023, UCB announced
In October 2023, the
In November 2023, UCB filed bimekizumab for the treatment of hidradenitis suppurativa (HS), a chronic, painful, and debilitating skin condition, with PMDA in
In December 2023, ZILBRYSQ® (zilucoplan) was approved in the European Union for the treatment of adults with gMG who are anti-AChRAb+. In September 2023, UCB received CHMP positive opinion for zilucoplan for the treatment of adults with gMG in
In December 2023, BIMZELX® was approved in
In early January 2024, RYSTIGGO® (rozanolixizumab) was approved in the European Union for the treatment of adults with generalized myasthenia gravis (gMG) who are anti-acetylcholine receptor (AChR) or anti-muscle-specific tyrosine kinase (MuSK) antibody positive. In November 2023, UCB received the CHMP positive opinion for rozanolixizumab for treatment of adults with generalized myasthenia gravis in
In February 2024, UCB announced that the
Pipeline Update
In November 2023, first patients were included in a phase 2a study with UCB0022. UCB0022 is designed to enhance the potency of endogenous dopamine 'when and where needed'. UCB0022 is an orally available, brain-penetrant, small molecule acting as a Dopamine-1 receptor positive allosteric modulator. UCB0022 could bring, as symptomatic treatment, significant positive impact on the quality of life of people who are suffering from Parkinson's symptoms despite an adequately dosed treatment without bothersome side effects that can result from Dopamine-receptor overstimulation. First results are expected in H1 in 2025.
During H2 2023, UCB9741 and UCB1381 progressed successfully and moved into Phase 2a status with first headline results expected in H2 2024. Atopic Dermatitis (AtD) is a common inflammatory skin disorder with higher prevalence rates among children. Despite evolving standard of care, unmet needs for moderate to severe AtD patients persist. Multiple pathways are believed to be the driver of pathobiology in AtD, as such UCB is developing two anti-bodies targeting distinct pathways.
All other clinical studies are continuing as planned, with headline results expected for 11 programs in 2024.
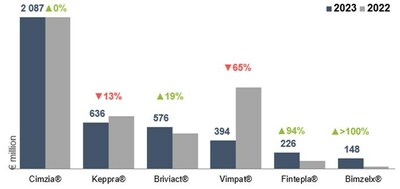
Net sales break-down by key products
Due to rounding, some financial data may not add up in the tables.
CIMZIA® (certolizumab pegol) reached more than 180 000 people living with inflammatory TNF-mediated diseases. CIMZIA® is showing a stronger growth than the anti-TNF market – based on differentiation. CIMZIA® is offering treatment for women of childbearing age across 6 indications and for rheumatoid arthritis patients with high rheum factor levels. In
€ million | 2023 | 2022 | Act | CER1 |
U.S. | 1 364 | 1 381 | -1 % | 2 % |
428 | 416 | 3 % | 3 % | |
39 | 51 | -24 % | -17 % | |
International markets | 257 | 237 | 8 % | 16 % |
Total Cimzia® | 2 087 | 2 085 | 0 % | 3 % |
VIMPAT® (lacosamide) was accessed by over 500 000 people living with epilepsy and is experiencing generic competition since March 2022 in the
€ million | 2023 | 2022 | Act | CER1 |
U.S. | 96 | 706 | -86 % | -86 % |
140 | 272 | -48 % | -48 % | |
83 | 68 | 22 % | 34 % | |
International markets | 75 | 77 | -3 % | 4 % |
Total Vimpat® | 394 | 1 124 | -65 % | -63 % |
KEPPRA® (levetiracetam) reached over 1.7 million people living with epilepsy. Net sales went down due to continued generic erosion in
€ million | 2023 | 2022 | Act | CER1 |
U.S. | 132 | 156 | -16 % | -13 % |
205 | 206 | -1 % | 0 % | |
97 | 149 | -35 % | -28 % | |
International markets | 202 | 217 | -7 % | 3 % |
Total Keppra® | 636 | 729 | -13 % | -8 % |
BRIVIACT® (brivaracetam), was used by 190 000 people living with epilepsy and showed significant growth in all regions it is available to patients. BRIVIACT® is currently under regulatory review in
€ million | 2023 | 2022 | Act | CER1 |
U.S. | 445 | 380 | 17 % | 20 % |
110 | 88 | 25 % | 25 % | |
International markets | 21 | 17 | 22 % | 27 % |
Total Briviact® | 576 | 485 | 19 % | 21 % |
FINTEPLA® (fenfluramine) reached more than 3 000 patients and their families living with seizures associated with rare epileptic syndromes - Dravet Syndrome (DS) and Lennox-Gastaut Syndrome (LGS). Partner Nippon Shinyaku in
€ million | 2023 | 2022 | Act | CER1 |
U.S. | 201 | 107 | 88 % | 93 % |
21 | 8 | > | > | |
1 | 1 | 54 % | 70 % | |
International markets | 3 | 1 | > | > |
Total Fintepla® | 226 | 116 | 94 % | 99 % |
BIMZELX® (bimekizumab) is available to people living with psoriasis in more than 40 countries, including the
€ million | 2023 | 2022 | Act | CER1 |
U.S. | 9 | - | N/A | N/A |
112 | 29 | > | > | |
16 | 4 | > | > | |
International markets | 12 | 2 | > | > |
Total Bimzelx® | 148 | 35 | > | > |
NAYZILAM® (midazolam) Nasal SprayCIV, a nasal rescue treatment for epilepsy seizure clusters in the
EVENITY® (romosozumab) since launch globally reached more than 600 000 women living with postmenopausal osteoporosis at high risk of fracture. Net sales in
RYSTIGGO® (rozanolixizumab-noli), a new treatment option for people living with generalized myasthenia gravis (gMG) was launched in the
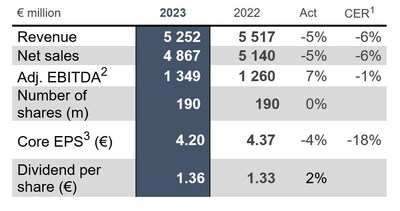
2023 FY financial highlights | ||||
Actual1 | Variance | |||
€ million | 2023 | 2022 | Actual rates | CER2 |
Revenue | 5 252 | 5 517 | -5 % | -6 % |
Net sales | 4 867 | 5 140 | -5 % | -6 % |
Royalty income and fees | 77 | 85 | -9 % | -7 % |
Other revenue | 308 | 292 | 5 % | 6 % |
Adjusted Gross Profit | 4 033 | 4 239 | -5 % | -6 % |
Gross Profit | 3 545 | 3 843 | -8 % | -9 % |
Marketing and selling expenses | -1 594 | -1 489 | 7 % | 10 % |
Research and development expenses | -1 630 | -1 670 | -2 % | -1 % |
General and administrative expenses | - 230 | - 225 | 2 % | 3 % |
Other operating income/expenses (-) | 566 | 216 | > | > |
Adjusted EBIT | 657 | 675 | -3 % | -15 % |
Impairment, restructuring and other income/expenses (-) | - 53 | - 90 | -41 % | -38 % |
EBIT (operating profit) | 604 | 585 | 3 % | -13 % |
Net financial expenses | - 163 | - 74 | > | > |
Profit before income taxes | 441 | 511 | -14 % | -27 % |
Income tax expenses | - 98 | - 91 | 8 % | 21 % |
Profit from continuing operations | 343 | 420 | -18 % | -35 % |
Profit/loss (-) from discontinued operations | 0 | - 2 | -100 % | -100 % |
Profit | 343 | 418 | -18 % | -34 % |
Attributable to UCB shareholders | 343 | 418 | -18 % | -34 % |
Adjusted EBITDA | 1 349 | 1 260 | 7 % | -1 % |
Capital expenditure (including intangible assets) | 316 | 371 | -15 % | |
Net debt (-) | -2 177 | -2 000 | 9 % | |
Operating cash flow from continuing operations | 761 | 1 119 | -32 % | |
Weighted average number of shares – non diluted (million) | 190 | 190 | 0 % | |
1.81 | 2.20 | -18 % | -34 % | |
EPS (€ per weighted average number of shares – non diluted) | ||||
Core EPS (€ per weighted average number of shares – non | 4.20 | 4.37 | -4 % | -18 % |
1 Due to rounding, some financial data may not add up in the tables included in this management report | ||||
"The statutory auditor has issued an unqualified report with no emphasis of matter paragraph dated 27 February 2024 on the company's consolidated accounts as of and for the year ended 31 December 2023, and has confirmed that the accounting data reported in the accompanying press release is consistent, in all material respects, with the accounts from which it has been derived."
Revenue in 2023 reached
Royalty income and fees were
Adjusted Gross profit (Gross Profit before "amortization of intangible assets linked to sales") was
Gross profit after "amortization of intangible assets linked to sales" reached
Operating expenses declined to
7% higher marketing and selling expenses of€ 1 594 million (+10% CER1) – focused reallocation and cost discipline allowed to invest behind the launches and pre-launch activities for UCB's growth drivers: Global FINTEPLA® launch activities in two indications, global BIMZELX® launch activities in up to four indications, global launch activities for RYSTIGGO® and ZILBRYSQ®.2% lower research and development expenses of€ 1 630 million (-1% CER1) reflect the continued investments in UCB's progressing R&D pipeline today encompassing 10 potential new treatment options in clinical studies for patients living with severe diseases in 5 phase 3 trials and 7 proof-of-concept (phase 2a) trials as well as ongoing earlier research activities. The R&D ratio reached31% in 2023 after30% in 2022 due to lower revenue.2% higher general and administrative expenses of€ 230 million (+3% CER)- Other operating income went up to
€ 566 million following€ 216 million in 2022 – driven by the net contribution of€ 368 million (+53% ) from EVENITY®. Other 'other operating income' was from the sale of a portfolio of established brands inEurope (€ 145 million ), in early 2023.
Underlying operational profitability – adjusted EBITDA2 – increased by
Total impairment, restructuring and other expenses decreased to
Net financial expenses went up to
Income tax expenses were
Profit amounted to
Core earnings per share, adjusted for the after-tax impact of to be adjusted items, the financial one-offs, the after-tax contribution from discontinued operations and the net amortization of intangibles linked to sales, reached
Dividend - The Board of Directors of UCB proposes a dividend of
Financial Guidance 2024 - The year 2024 will be marked by intense ongoing global launches of the UCB growth drivers, BIMZELX®, RYSTIGGO®, ZILBRYSQ® and FINTEPLA® as well as EVENITY®.
For 2024, UCB is aiming for an increase of revenues to the range of
UCB will accelerate investments in launches around the globe to offer potential new solutions for people living with severe diseases and remains committed to invest into research and development advancing its late-stage and early development pipeline. At the same time, UCB will continue to be cost disciplined and, as in 2023, to actively manage the tail of its portfolio. Underlying profitability, adjusted EBITDA, is expected in the range of
The figures for the financial guidance 2024 as mentioned above are calculated on the same basis as the actual figures for 2023.
Guidance for 2025: UCB confirms its growth ambition for 2025 based on the strong product portfolio and the strong growth drivers. Revenue in 2025 is expected to reach at least
Find the financial reports on UCB website: http://www.ucb.com/investors/Download-center
Today, UCB will host a conference call/video webcast at 08.00 (EST) / 13.00 (GMT) / 14.00 (CET)
Register here: https://www.ucb.com/investors
For further information, contact UCB:
Investor Relations | Global Communications | |
Antje Witte | Julien Bayet | Laurent Schots, Media Relations |
Check out our IR App on the App Store and Google Play
About UCB
UCB,
Forward looking statements
This press release contains forward-looking statements, including, without limitation, statements containing the words "potential", "believes", "anticipates", "expects", "intends", "plans", "seeks", "estimates", "may", "will", "continue" and similar expressions. These forward-looking statements are based on current plans, estimates and beliefs of management. All statements, other than statements of historical facts, are statements that could be deemed forward-looking statements, including estimates of revenues, operating margins, capital expenditures, cash, other financial information, expected legal, arbitration, political, regulatory or clinical results or practices and other such estimates and results. By their nature, such forward-looking statements are not guaranteeing future performance and are subject to known and unknown risks, uncertainties, and assumptions which might cause the actual results, financial condition, performance or achievements of UCB, or industry results, to be materially different from any future results, performance, or achievements expressed or implied by such forward-looking statements contained in this press release.
Important factors that could result in such differences include but are not limited to: global spread and impacts of wars and pandemics, changes in general economic, business and competitive conditions, the inability to obtain necessary regulatory approvals or to obtain them on acceptable terms or within expected timing, costs associated with research and development, changes in the prospects for products in the pipeline or under development by UCB, effects of future judicial decisions or governmental investigations, safety, quality, data integrity or manufacturing issues; potential or actual data security and data privacy breaches, or disruptions of our information technology systems, product liability claims, challenges to patent protection for products or product candidates, competition from other products including biosimilars, changes in laws or regulations, exchange rate fluctuations, changes or uncertainties in tax laws or the administration of such laws, and hiring and retention of its employees. There is no guarantee that new product candidates will be discovered or identified in the pipeline, or that new indications for existing products will be developed and approved. Movement from concept to commercial product is uncertain; preclinical results do not guarantee safety and efficacy of product candidates in humans. So far, the complexity of the human body cannot be reproduced in computer models, cell culture systems or animal models. The length of the timing to complete clinical trials and to get regulatory approval for product marketing has varied in the past and UCB expects similar unpredictability going forward. Products or potential products which are the subject of partnerships, joint ventures or licensing collaborations may be subject to disputes between the partners or may prove to be not as safe, effective or commercially successful as UCB may have believed at the start of such partnership. UCB's efforts to acquire other products or companies and to integrate the operations of such acquired companies may not be as successful as UCB may have believed at the moment of acquisition. Also, UCB or others could discover safety, side effects or manufacturing problems with its products and/or devices after they are marketed. The discovery of significant problems with a product similar to one of UCB's products that implicate an entire class of products may have a material adverse effect on sales of the entire class of affected products. Moreover, sales may be impacted by international and domestic trends toward managed care and health care cost containment, including pricing pressure, political and public scrutiny, customer and prescriber patterns or practices, and the reimbursement policies imposed by third-party payers as well as legislation affecting biopharmaceutical pricing and reimbursement activities and outcomes. Finally, a breakdown, cyberattack or information security breach could compromise the confidentiality, integrity and availability of UCB's data and systems.
Given these uncertainties, you are cautioned not to place any undue reliance on such forward-looking statements. These forward-looking statements are made only as of the date of this press release, and do not reflect any potential impacts from the evolving conflicts, wars, pandemics, as well as any other adversity, unless indicated otherwise. The company continues to follow the development diligently to assess the financial significance of these events, as the case may be, to UCB.
UCB expressly disclaims any obligation or duty to update any forward-looking statements in this press release, either to confirm the actual results or to report or reflect any change in its forward-looking statements with regard thereto or any change in events, conditions or circumstances on which any such statement is based, unless such statement is required pursuant to applicable laws and regulations.
1 | CER = constant exchange rates |
2 | adj. EBITDA = adjusted Earnings Before Interest, Taxes, Depreciation and Amortization charges |
3 | Core EPS = core earnings per share |
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/ucb-on-growth-path-for-a-decade-plus-302073984.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/ucb-on-growth-path-for-a-decade-plus-302073984.html
SOURCE UCB