Vivani Medical Announces Rapid Advancement of NPM-139, a Novel Semaglutide Implant, Following Positive Weight Loss Data from an Ongoing Preclinical Study of NPM-139 and Promising Results from the LIBERATE-1 Phase 1 Clinical Study of NPM-115
Vivani Medical (NASDAQ:VANI) reported significant progress in its drug implant development programs. The company announced positive results from two key studies: the LIBERATE-1 Phase 1 clinical study of NPM-115 (exenatide implant) and preclinical data for NPM-139 (semaglutide implant).
The LIBERATE-1 study met its primary objectives, demonstrating safety and tolerability of the NanoPortal™ implant technology. More notably, the NPM-139 preclinical study showed approximately 20% weight loss maintained for over 6 months with a single implant administration, suggesting potential for annual dosing.
Based on these results, Vivani is prioritizing the development of NPM-139, with clinical trials expected to begin in 2026. The decision is supported by the strong commercial performance of semaglutide-based products, which generated over $29B in sales in 2024.
Vivani Medical (NASDAQ:VANI) ha annunciato importanti progressi nei suoi programmi di sviluppo di impianti farmaceutici. L'azienda ha comunicato risultati positivi da due studi chiave: lo studio clinico di Fase 1 LIBERATE-1 sull'NPM-115 (impianto di exenatide) e i dati preclinici per NPM-139 (impianto di semaglutide).
Lo studio LIBERATE-1 ha raggiunto i suoi obiettivi primari, dimostrando la sicurezza e la tollerabilità della tecnologia impiantabile NanoPortal™. Ancora più rilevante, lo studio preclinico su NPM-139 ha evidenziato una perdita di peso di circa il 20% mantenuta per oltre 6 mesi con una singola somministrazione dell’impianto, suggerendo la possibilità di una somministrazione annuale.
Alla luce di questi risultati, Vivani sta dando priorità allo sviluppo di NPM-139, con l’avvio delle sperimentazioni cliniche previsto per il 2026. La decisione è supportata dall’ottima performance commerciale dei prodotti a base di semaglutide, che hanno generato oltre 29 miliardi di dollari di vendite nel 2024.
Vivani Medical (NASDAQ:VANI) informó avances significativos en sus programas de desarrollo de implantes farmacéuticos. La compañía anunció resultados positivos de dos estudios clave: el estudio clínico de Fase 1 LIBERATE-1 de NPM-115 (implante de exenatida) y datos preclínicos para NPM-139 (implante de semaglutida).
El estudio LIBERATE-1 cumplió con sus objetivos principales, demostrando la seguridad y tolerabilidad de la tecnología de implante NanoPortal™. Más notablemente, el estudio preclínico de NPM-139 mostró una pérdida de peso aproximada del 20% mantenida por más de 6 meses con una sola administración del implante, lo que sugiere potencial para una dosificación anual.
Basándose en estos resultados, Vivani está priorizando el desarrollo de NPM-139, con ensayos clínicos previstos para comenzar en 2026. La decisión está respaldada por el sólido desempeño comercial de los productos basados en semaglutida, que generaron más de $29 mil millones en ventas en 2024.
Vivani Medical (NASDAQ:VANI)는 약물 임플란트 개발 프로그램에서 중요한 진전을 보고했습니다. 회사는 두 가지 주요 연구 결과를 발표했는데, 이는 LIBERATE-1 1상 임상 연구인 NPM-115(엑세나타이드 임플란트)와 NPM-139(세마글루타이드 임플란트)의 전임상 데이터입니다.
LIBERATE-1 연구는 주요 목표를 달성하여 NanoPortal™ 임플란트 기술의 안전성과 내약성을 입증했습니다. 특히 NPM-139 전임상 연구에서는 단일 임플란트 투여로 약 20% 체중 감량이 6개월 이상 유지됨을 보여 연간 투여 가능성을 시사했습니다.
이 결과를 바탕으로 Vivani는 NPM-139 개발을 우선시하며, 임상 시험은 2026년에 시작될 예정입니다. 이 결정은 2024년에 290억 달러 이상의 매출을 기록한 세마글루타이드 기반 제품의 강력한 상업적 성과에 힘입은 것입니다.
Vivani Medical (NASDAQ:VANI) a annoncé des progrès significatifs dans ses programmes de développement d’implants médicamenteux. La société a communiqué des résultats positifs issus de deux études clés : l'étude clinique de phase 1 LIBERATE-1 sur le NPM-115 (implant d’exénatide) et les données précliniques pour le NPM-139 (implant de sémaglutide).
L’étude LIBERATE-1 a atteint ses objectifs principaux, démontrant la sécurité et la tolérabilité de la technologie d’implant NanoPortal™. Plus notablement, l'étude préclinique sur le NPM-139 a montré une perte de poids d’environ 20 % maintenue pendant plus de 6 mois après une administration unique de l’implant, suggérant un potentiel pour une administration annuelle.
Sur la base de ces résultats, Vivani priorise le développement du NPM-139, avec des essais cliniques prévus pour commencer en 2026. Cette décision est soutenue par la forte performance commerciale des produits à base de sémaglutide, qui ont généré plus de 29 milliards de dollars de ventes en 2024.
Vivani Medical (NASDAQ:VANI) meldete bedeutende Fortschritte in seinen Programmen zur Entwicklung von Arzneimittelimplantaten. Das Unternehmen gab positive Ergebnisse aus zwei wichtigen Studien bekannt: der LIBERATE-1 Phase-1-Studie zu NPM-115 (Exenatid-Implantat) und präklinische Daten zu NPM-139 (Semaglutid-Implantat).
Die LIBERATE-1-Studie erreichte ihre primären Ziele und zeigte die Sicherheit und Verträglichkeit der NanoPortal™-Implantattechnologie. Besonders bemerkenswert ist, dass die präklinische Studie zu NPM-139 einen Gewichtsverlust von etwa 20 % zeigte, der über 6 Monate mit einer einzigen Implantatverabreichung aufrechterhalten wurde, was auf eine mögliche jährliche Dosierung hindeutet.
Auf Basis dieser Ergebnisse priorisiert Vivani die Entwicklung von NPM-139, und klinische Studien sollen im Jahr 2026 beginnen. Diese Entscheidung wird durch die starke kommerzielle Leistung der auf Semaglutid basierenden Produkte gestützt, die im Jahr 2024 über 29 Milliarden US-Dollar Umsatz erzielten.
- NPM-139 preclinical study demonstrated 20% weight loss maintained for over 6 months with single implant
- LIBERATE-1 Phase 1 study met primary objectives with positive safety and tolerability profile
- Semaglutide-based reference products generated over $29B in sales in 2024
- Technology shows potential for annual dosing, offering competitive advantage in the weight management market
- Clinical development of NPM-139 not expected to begin until 2026
- Current data only supports bi-annual dosing, with annual dosing still a future target
Insights
Vivani's implant technology shows promising results with 20% weight loss from semaglutide implant, pivoting development toward the lucrative GLP-1 market.
Vivani Medical has presented a strategic pivot that significantly strengthens their competitive positioning in the booming GLP-1 market. Their NanoPortal™ technology has now been validated in humans through the LIBERATE-1 Phase 1 trial of NPM-115 (exenatide implant), which successfully met safety and tolerability endpoints. The absence of gastrointestinal adverse events and lack of initial drug burst release are particularly noteworthy, as these are common challenges with GLP-1 medications.
The more compelling development is their preclinical data for NPM-139, a semaglutide implant that achieved
Strategically, the company's decision to prioritize semaglutide over exenatide is sound. Semaglutide has already demonstrated superior efficacy in weight management compared to exenatide and generated over
The ability to maintain consistent drug levels without peaks and troughs could potentially improve both efficacy and tolerability compared to injectable products. If NPM-139 advances successfully into human trials in 2026 and maintains this performance profile, it could represent a meaningful advance in GLP-1 therapy administration.
LIBERATE-1, the first-in-human application of Vivani’s NanoPortalTM implant technology, showed a positive safety and tolerability profile, along with encouraging performance data for NPM-115 that met the study’s primary objectives
New NPM-139 (semaglutide implant) preclinical feasibility data showed approximately
Based on these data, Vivani prioritizes the advancement of NPM-139, with clinical development expected to begin in 2026, pending regulatory clearance
ALAMEDA, Calif., Aug. 05, 2025 (GLOBE NEWSWIRE) -- Vivani Medical, Inc. (Nasdaq: VANI) (“Vivani” or the “Company”), a clinical-stage, biopharmaceutical company developing miniature, ultra long-acting drug implants, today reported results from the LIBERATE-1 clinical study, the Phase 1 study of the exenatide GLP-1 implant NPM-115 representing the first-in-human test of the Company’s proprietary NanoPortal™ implant technology.
The Company also reported new feasibility data for NPM-139 (semaglutide implant) from an ongoing preclinical study, supporting prioritization of the semaglutide implant in the Company’s pipeline and clinical development strategy. Semaglutide is the active ingredient in blockbuster drug products Ozempic®, Wegovy®, and Rybelsus®.
Vivani Chief Executive Officer Adam Mendelsohn, Ph.D. stated, “We are very pleased to report that LIBERATE-1, our Phase 1 study in obese and overweight subjects and the first clinical application of NanoPortal technology, achieved its primary objectives. The results support the general safety and tolerability profile of the device and continued development with higher dose configurations, which we expect to produce clinically relevant weight management effects.”
“In addition to the LIBERATE-1 results, we are very excited to report new preclinical feasibility data with our semaglutide implant candidate NPM-139, under development for chronic weight management which have shown approximately
The decision to prioritize NPM-139 is supported by several factors. These include the Company’s belief that the development timelines for the NPM-115 and NPM-139 programs are comparable, the increased confidence in NPM-139 due to the fact that semaglutide products have already established compelling weight loss data in humans, and the strong commercial performance of semaglutide-based products, including Ozempic, Wegovy, and Rybelsus, which have generated over
LIBERATE-1 Study Results
The LIBERATE-1 Phase 1 study successfully met its primary objectives, which were to evaluate the NPM-115 implant’s safety and tolerability profile and to characterize the pharmacokinetic (PK) profile of the implant over a 9-week duration. Throughout the study, the implant was generally well tolerated, and drug release from the implant without any clinically meaningful burst was supported by PK analysis and by the absence of gastrointestinal adverse events in subjects with the implant. No serious adverse events were observed in the study. The release profile observed from the implants over 9 weeks provides encouragement regarding the potential for this technology to provide durable delivery over the 6-month duration that has already been established in preclinical studies of both NPM-115 and NPM-139.
This study paves the way for future clinical development of the implant technology not just for exenatide (NPM-115 and NPM-119) but also for semaglutide (NPM-139) and any other application of NanoPortal technology that Vivani may pursue in the future.
Semaglutide Implant Preclinical Feasibility Data
In an ongoing preclinical study, substantial progress in the development of a miniature, ultra long-acting, semaglutide implant, NPM-139 has been established by showing weight loss from a single administration for over 231 days.
NPM-139 (Semaglutide Implant)
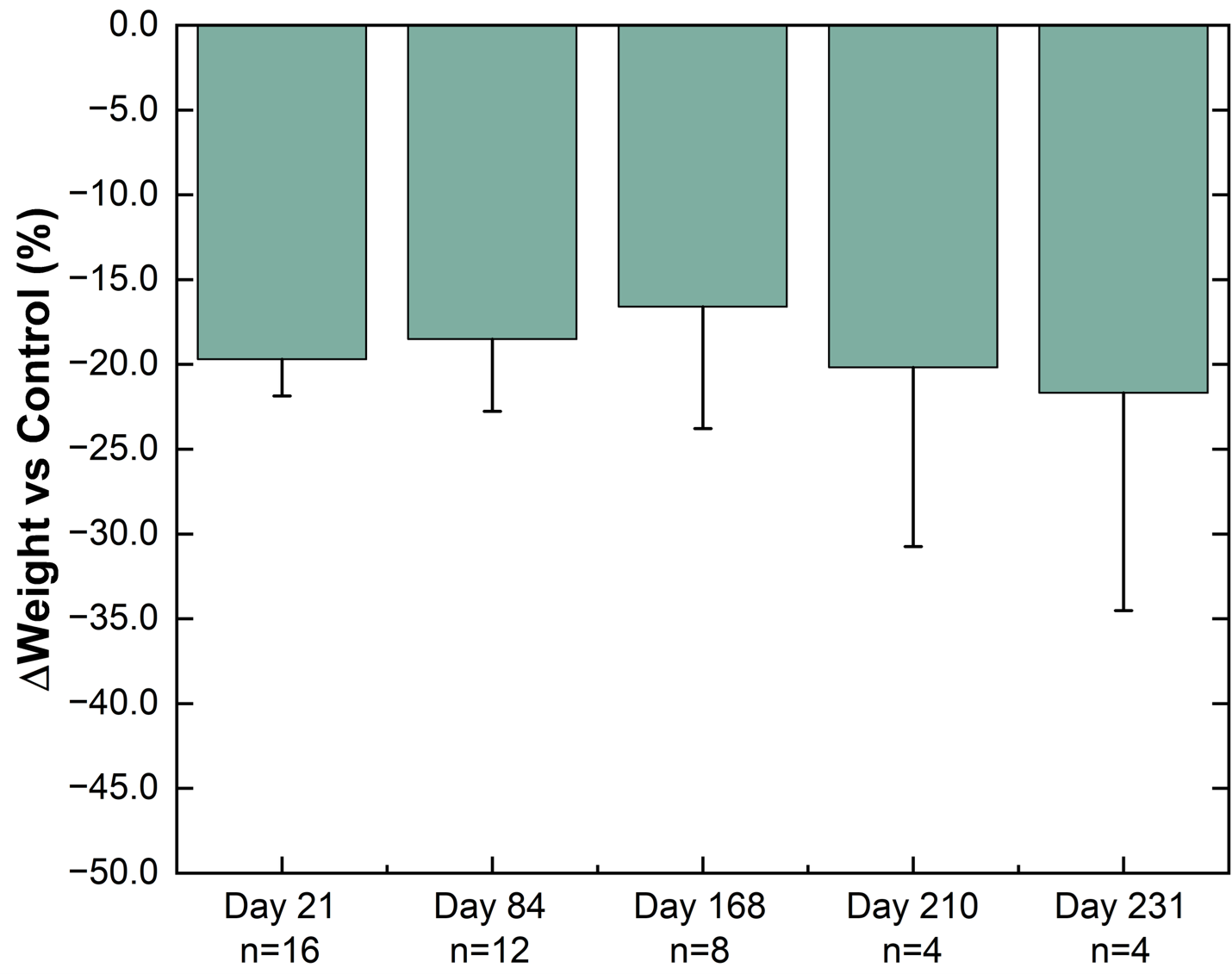
Weight difference versus control group in healthy Sprague-Dawley rats. The percentage weight change from baseline for NPM-139 (semaglutide implant) corrected to control (sham implant). Implants from 4 animals were removed on each of Day 21, Day 84, and Day 168 for characterization. Values are mean ± standard error.
While the emerging preclinical data on the Company’s semaglutide implant currently supports the initial target profile of bi-annual dosing, the Company continues to anticipate that a semaglutide implant candidate may be able to support annual dosing in the future. Vivani’s near-term efforts are focused on completion of PK optimization activities and preparation of data to enable the submission of an Investigational New Drug application for NPM-139.
About Vivani Medical, Inc.
Leveraging its proprietary NanoPortal™ platform, Vivani develops biopharmaceutical implants designed to deliver drug molecules steadily over extended periods of time with the goal of guaranteeing adherence and improving patient tolerance to their medication. Vivani’s priority product candidate, NPM-139, is a miniature, six-month, subdermal, GLP-1 (semaglutide) implant under development for chronic weight management in obese or overweight subjects. NPM-139 has the added potential for once-yearly dosing. Vivani’s emerging pipeline also includes NPM-115 (exenatide implant) for chronic weight management in obese and overweight individuals, and NPM-119, an exenatide implant program for the treatment of type-2 diabetes. The Company is also considering another semaglutide implant for the treatment of type 2 diabetes. These NanoPortal implants are designed to provide patients with the opportunity to realize the full potential benefit of their medication by avoiding the numerous challenges associated with the daily or weekly administration of orals and injectables, including tolerability issues and loss of efficacy. Medication non-adherence occurs when patients do not take their medication as prescribed. This affects an alarming number of patients, approximately
Forward-Looking Statements
This press release contains certain “forward-looking statements” within the meaning of the “safe harbor” provisions of the US Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified by words such as: “target,” “believe,” “expect,” “will,” “may,” “anticipate,” “estimate,” “would,” “positioned,” “future,” and other similar expressions that in this press release, including statements regarding Vivani’s business, products in development, including the therapeutic potential thereof, the planned development therefor, the completion of the LIBERATE-1 Phase 1 study and reporting of study results, Vivani’s emerging development plans for NPM-139, NPM-115, NPM-119 or Vivani’s plans with respect its technology, strategy, cash position and financial runway. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on Vivani’s current beliefs, expectations, and assumptions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of Vivani’s control. Actual results and outcomes may differ materially from those indicated in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause actual results and outcomes to differ materially from those indicated in the forward-looking statements include, among others, risks related to the development and commercialization of Vivani’s products, including NPM-139, NPM-115, and NPM-119; delays and changes in the development of Vivani’s products, including as a result of applicable laws, regulations and guidelines, potential delays in submitting and receiving regulatory clearance or approval to conduct Vivani’s development activities, including Vivani’s ability to commence clinical development of NPM-139; risks related to the initiation, enrollment and conduct of Vivani’s planned clinical studies and the results therefrom; or Vivani’s history of losses and Vivani’s ability to access additional capital or otherwise fund Vivani’s business. There may be additional risks that the Company considers immaterial, or which are unknown. A further list and description of risks and uncertainties can be found in the Company’s most recent Annual Report on Form 10-K filed with the U.S. Securities and Exchange Commission on March 31, 2025, as updated by the Company’s subsequent Quarterly Reports on Form 10-Q. Any forward-looking statement made by Vivani in this press release is based only on information currently available to the Company and speaks only as of the date on which it is made. The Company undertakes no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of added information, future developments or otherwise, except as required by law.
Company Contact:
Donald Dwyer
Chief Business Officer
info@vivani.com
(415) 506-8462
Investor Relations Contact:
Jami Taylor
Investor Relations Advisor
investors@vivani.com
(415) 506-8462
Media Contact:
Mark Corbae
ICR Healthcare
Mark.Corbae@ICRHealthcare.com
(203) 682-8288
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/8a049bc2-9622-4156-b325-dc73a1f1b9d1.









