UTime Limited Launches Smartwatch with Integrated Blood Pressure Monitoring
Rhea-AI Summary
UTime (Nasdaq: WTO) launched a smartwatch with integrated blood pressure monitoring on Oct 28, 2025, expanding its health tech product lineup.
The device uses the oscillometric method with an integrated micro air pump and high-precision pressure sensor, and it holds a China NMPA Class II Medical Device Registration (Registration Certificate No. Guangdong Instrument Note No. 20162070928). The watch also reports heart rate, blood oxygen saturation, sleep analysis, and multiple sports modes while emphasizing wearing comfort and interactive features like voice reminders.
Positive
- China NMPA Class II medical registration (Reg. No. 20162070928)
- Uses market-validated oscillometric blood pressure method
- Integrated micro air pump and high-precision pressure sensor
Negative
- None.
News Market Reaction
On the day this news was published, WTO gained 2.39%, reflecting a moderate positive market reaction. Argus tracked a peak move of +15.2% during that session. Argus tracked a trough of -11.7% from its starting point during tracking. Our momentum scanner triggered 18 alerts that day, indicating notable trading interest and price volatility. This price movement added approximately $170K to the company's valuation, bringing the market cap to $7M at that time. Trading volume was very high at 3.5x the daily average, suggesting strong buying interest.
Data tracked by StockTitan Argus on the day of publication.
SHENZHEN, China, Oct. 28, 2025 (GLOBE NEWSWIRE) -- UTime Limited (Nasdaq: WTO), a global technology company engaged in the design and development of mobile devices, today announced the launch of a smartwatch with integrated blood pressure monitoring functionality, further expanding its product portfolio in the health technology sector.
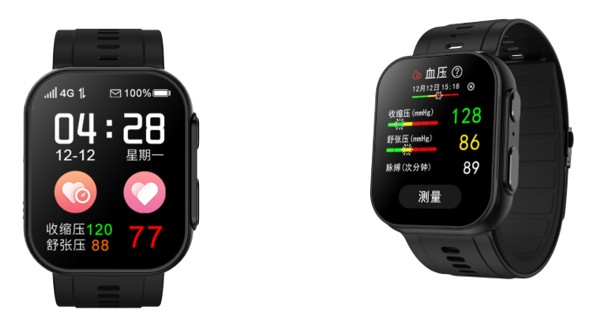
The product utilizes the Oscillo metric method, a market-validated measurement principle, and integrates a micro air pump and high-precision pressure sensor to provide users with a convenient blood pressure monitoring experience. It has obtained China NMPA Class II Medical Device Registration Certificate (Registration Certificate No.: Guangdong Instrument Note No. 20162070928). In addition to its core blood pressure monitoring feature, the watch also provides multi-dimensional health data references, including heart rate, blood oxygen saturation, sleep analysis, and multiple sports mode tracking. The product design balances wearing comfort with daily practicality and includes interactive features such as voice reminders.
Mr. Hengcong Qiu, Chief Executive Officer of UTime Limited, stated, "We focus on integrating practical health monitoring functions with mobile smart devices. The launch of this watch represents our efforts to meet the growing user demand for health management."
About UTime Limited
Operating under the NASDAQ ticker WTO, UTime Limited is engaged in the design, development, production, sales and brand operation of mobile devices in China and globally. The company aims to provide cost-effective products and serves a broad customer base.
Safe Harbor Statement
This press release contains forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended. All statements other than statements of historical fact are forward-looking statements. These statements involve known and unknown risks, uncertainties and other factors that may cause the Company’s actual results, performance or achievements to be materially different from any future results, performances or achievements expressed or implied by the forward-looking statements. For additional risk factors, please review UTime Limited’s Annual Report on Form 20-F and other SEC filings. Forward-looking statements are made only as of the date of this release.The Company undertakes no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required under applicable law.
Contact:
qhengcong@utimemobile.com
UTime Limited
7th Floor, Building 5A
Shenzhen Software Industry Base, Nanshan District
Shenzhen, People’s Republic of China 518061
Tel: (86) 755 86512266
Attachment









