Anavex Life Sciences Announces Continued Long-Term Benefit from Oral Blarcamesine Compared to Decline Observed in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) Control Group
Anavex Life Sciences (Nasdaq: AVXL) reported long-term clinical benefit for oral blarcamesine in early Alzheimer’s disease versus externally matched ADNI controls over 144 weeks.
Key outcomes: ADAS-Cog13 mean change differences were −2.68 points at 48 weeks, −6.41 points at 96 weeks, and −12.78 points at 144 weeks (all p < 0.0001). The company reports an estimated 77.4 weeks (≈17.8 months) of “time saved” versus ADNI. Blarcamesine showed a favorable safety profile with no treatment-related deaths reported.
The release also reiterates a proposed mechanism—SIGMAR1 activation restoring impaired autophagy upstream of amyloid and tau—and notes the data will be published and presented at international Alzheimer’s conferences. The drug remains investigational with no guarantee of regulatory approval.
Anavex Life Sciences (Nasdaq: AVXL) ha riportato benefici clinici a lungo termine per la forma orale blarcamesine nella fase iniziale di Alzheimer rispetto a controlli esternamente abbinati ADNI su 144 settimane.
Risultati chiave: la variazione media di ADAS-Cog13 è stata di −2,68 punti a 48 settimane, −6,41 punti a 96 settimane e −12,78 punti a 144 settimane (tutti p < 0,0001). L'azienda riporta una stima di 77,4 settimane (≈17,8 mesi) di “tempo risparmiato” rispetto all'ADNI. Blarcamesine ha mostrato un profilo di sicurezza favorevole con nessuna morte correlata al trattamento riportata.
Il comunicato riprende anche un meccanismo proposto—l'attivazione SIGMAR1 che ripristina l'autofagia compromessa a monte di amiloide e tau—e nota che i dati saranno pubblicati e presentati a conferenze internazionali sull'Alzheimer. Il farmaco rimane investigazionale senza alcuna garanzia di approvazione regolatoria.
Anavex Life Sciences (Nasdaq: AVXL) informó beneficios clínicos a largo plazo para blarcamesine oral en la enfermedad de Alzheimer en etapa temprana frente a controles externos pareados con ADNI durante 144 semanas.
Resultados clave: las diferencias en el cambio medio de ADAS-Cog13 fueron de −2,68 puntos a las 48 semanas, −6,41 puntos a las 96 semanas y −12,78 puntos a las 144 semanas (todos p < 0,0001). La empresa reporta unas ≈77,4 semanas (≈17,8 meses) de “tiempo ahorrado” frente a ADNI. Blarcamesine mostró un perfil de seguridad favorable sin muertes relacionadas con el tratamiento reportadas.
El comunicado también reitera un mecanismo propuesto—la activación de SIGMAR1 que restablece la autofagia deteriorada aguas arriba de la beta-amiloide y tau—y señala que los datos se publicarán y presentarán en conferencias internacionales sobre Alzheimer. El medicamento sigue siendo investigacional y no hay garantía de aprobación regulatoria.
Anavex Life Sciences (Nasdaq: AVXL)는 144주 동안 외부 매칭된 ADNI 대조군 대비 초기 알츠하이머 병에서 경구용 블라카메신에 대한 장기 임상 이점을 보고했습니다.
주요 결과: ADAS-Cog13 평균 변동 차이는 48주에 −2.68점, 96주에 −6.41점, 144주에 −12.78점이었습니다(모두 p < 0.0001). 회사는 ADNI에 비해 약 77.4주(약 17.8개월) “절약된 시간”을 보고합니다. 블라카메신은 치료와 관련된 사망이 보고되지 않은 안전성 프로파일을 보였습니다.
발표자료는 또한 제안된 기전—SIGMAR1 활성화가 아밀로이드와 타우 앞선 단계에서 자가포식(autophagy)을 복원한다—을 재확인하고, 데이터가 국제 알츠하이머 학회에서 발표 및 게재될 것이라고 밝힙니다. 이 약물은 연구 중이며 규제 승인의 보장은 없습니다.
Anavex Life Sciences (Nasdaq: AVXL) a rapporté un bénéfice clinique à long terme pour le blarcamesine oral chez les patients à un stade précoce d’Alzheimer par rapport à des témoins ADNI appariés externes sur 144 semaines.
Résultats clés : les différences moyennes de changement de ADAS-Cog13 étaient de −2,68 points à 48 semaines, −6,41 points à 96 semaines et −12,78 points à 144 semaines (tous p < 0,0001). L’entreprise rapporte environ 77,4 semaines (≈17,8 mois) de “temps gagné” par rapport à ADNI. Le blarcamesine a montré un profil de sécurité favorable avec aucune mort liée au traitement signalée.
Le communiqué rappelle également un mécanisme proposé—l’activation de SIGMAR1 restaurant l’autophagie en amont de l’amyloïde et de tau—et précise que les données seront publiées et présentées lors de conférences internationales sur Alzheimer. Le médicament reste expérimental et aucune garantie d’approbation réglementaire.
Anavex Life Sciences (Nasdaq: AVXL) berichtete über langfristigen klinischen Nutzen von oraler blarcamesine bei frühem Alzheimer im Vergleich zu extern gematchten ADNI-Kontrollen über 144 Wochen.
Schlüsselresultate: Die Unterschiede in der mittleren Veränderung des ADAS-Cog13 betrugen −2,68 Punkte nach 48 Wochen, −6,41 Punkte nach 96 Wochen und −12,78 Punkte nach 144 Wochen (alle p < 0,0001). Das Unternehmen berichtet eine geschätzte 77,4 Wochen (≈17,8 Monate) “gesparte Zeit” gegenüber ADNI. Blarcamesine zeigte ein günstiges Sicherheitsprofil mit keinen behandlungsbedingten Todesfällen.
Die Veröffentlichung bekräftigt auch einen vorgeschlagenen Mechanismus—SIGMAR1-Aktivierung, die die Autophagie upstream von Amyloid und Tau wiederherstellt—und weist darauf hin, dass die Daten veröffentlicht und auf internationalen Alzheimer-Konferenzen präsentiert werden. Das Medikament bleibt investigativ und es gibt keine Garantie für eine behördliche Zulassung.
Anavex Life Sciences (Nasdaq: AVXL) أبلغت عن فائدة سريرية طويلة الأمد لدواء blarcamesine الفموي في مرض الزهايمر المبكر مقارنةً بشواهد ADNI مطابقة خارجيًا لمدة 144 أسبوعًا.
النتائج الرئيسية: التغير المتوسط في ADAS-Cog13 كان −2.68 نقطة عند 48 أسبوعًا، −6.41 نقطة عند 96 أسبوعًا، و−12.78 نقطة عند 144 أسبوعًا (جميعها < 0.0001). تفيد الشركة بوجود ما يقدّر 77.4 أسبوعًا (حوالي 17.8 شهرًا) من “الوقت الذي تم توفيره” مقارنة بـ ADNI. أظهر/blarcamesine ملف سلامة مفضلاً مع عدم وجود وفيات مرتبطة بالعلاج تم الإبلاغ عنها.
يؤكد البيان أيضًا آلية مقترحة—تفعيل SIGMAR1 يعيد تنشيط الأوتوفاجي المتضرر أعلى الأميلويد والتاو—ويذكر أن البيانات ستُنشر وتعرض في مؤتمرات عالمية عن الزهايمر. الدواء لا يزال قيد البحث ولا توجد ضمانات للموافقة التنظيمية.
Anavex Life Sciences (Nasdaq: AVXL) 报告了口服 blarcamesine 在早期阿尔茨海默病中相对于外部匹配的 ADNI 对照组在144周内的长期临床获益。
关键结果:ADAS-Cog13 的平均变化差异在48周为 −2.68 点,96周为 −6.41 点,144周为 −12.78 点(均 p < 0.0001)。公司报告相对于 ADNI 估计有 77.4 周(约17.8个月)的“节省时间”。Blarcamesine 展现出有利的安全性特征,未报告任何与治疗相关的死亡。
新闻稿还重申一个拟议的机制——SIGMAR1 激活在淀粉样蛋白和 tau 上游恢复受损的自噬作用——并指出数据将发表于并在国际阿尔茨海默病会议上发表。该药物仍在研究中,不能保证监管批准。
- ADAS-Cog13 −2.68 mean change vs ADNI at 48 weeks (p < 0.0001)
- ADAS-Cog13 −6.41 mean change vs ADNI at 96 weeks (p < 0.0001)
- ADAS-Cog13 −12.78 mean change vs ADNI at 144 weeks (p < 0.0001)
- 77.4 weeks (≈17.8 months) of reported Alzheimer’s ‘time saved’ vs ADNI
- Favorable safety profile with no treatment-related deaths reported
- Blarcamesine is investigational and there is no guarantee it will gain regulatory approval
Insights
Long-term Phase IIb/III data show sustained cognitive benefit and 77.4 weeks of "time saved" with oral blarcamesine versus ADNI controls.
Anavex reports a sustained and increasing separation on ADAS-Cog13 versus an externally matched ADNI control group: mean differences of −2.68 points at 48 weeks, −6.41 points at 96 weeks, and −12.78 points at 144 weeks, each with p < 0.0001. The company quantifies this as 77.4 weeks of "time saved" in the ITT population and states there were no treatment-related deaths reported, which supports a tolerability claim for the study population.
The mechanistic claim is that blarcamesine restores impaired autophagy via SIGMAR1 activation, described as occurring upstream of amyloid‑beta and tau, with supportive in vitro and in vivo signals cited. The clinical data show sustained cognitive separation versus an external natural history control over
Watch for conference presentations and the planned publication to see comparator matching methodology, attrition handling, exact safety tables, and pre-specified versus post-hoc analyses; those disclosures will likely appear in the near term around forthcoming Alzheimer’s conferences and the manuscript timeline. Confirmatory regulatory‑grade randomized comparisons or formal regulator feedback remain the next material items to validate clinical and approval relevance.
ADAS-Cog13 difference −12.78 (P < 0.0001) with oral blarcamesine treatment compared to ADNI control group at Week 144
77.4 Weeks (17.8 Months) ‘time saved’ with oral blarcamesine compared to ADNI
Restoring impaired Autophagy − preceding amyloid-beta and tau
NEW YORK, Oct. 29, 2025 (GLOBE NEWSWIRE) -- Anavex Life Sciences Corp. (“Anavex” or the “Company”) (Nasdaq: AVXL), a clinical-stage biopharmaceutical company focused on developing innovative treatments for Alzheimer's disease, Parkinson's disease, schizophrenia, neurodevelopmental, neurodegenerative, and rare diseases, including Rett syndrome, and other central nervous system (CNS) disorders, today announced new findings for blarcamesine, an oral small molecule for the potential treatment of early Alzheimer’s disease.
New data demonstrate continued long-term benefit from oral blarcamesine compared to decline observed in the Alzheimer’s Disease Neuroimaging Initiative (ADNI)1 control group.
Externally matched control participants from the ADNI database were compared with participants over the 144-week period of ANAVEX2-73-AD-004 and its ATTENTION-AD (ANAVEX2-73-AD-EP-004) open-label extension (OLE) Phase IIb/III trial.2 For ADAS-Cog13, total score ranges from 0 to 85 with higher scores indicating increased cognitive impairment.
In the intent-to-treat (ITT) population, significantly less cognitive decline was observed for the blarcamesine participants compared to the ADNI control group at 48 weeks with a significant, and clinically meaningful difference in mean change from baseline ADAS-Cog13 total score of −2.68 points (p < 0.0001).3
Over the course of the open-label extension study at time point 96 weeks, these two groups diverged sharply, with statistically significant differences in mean change in ADAS-Cog13 total score at 96 weeks of −6.41 points (p < 0.0001). The difference between groups continues to increase at 144 weeks (ADAS-Cog13 total score difference of −12.78 points; p < 0.0001).
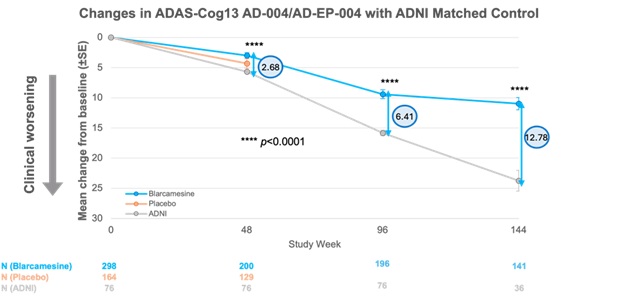
The results provide evidence of the significant beneficial therapeutic effect of blarcamesine, which positively separates from the ADNI control group with duration of treatment.
Alzheimer's disease ‘time saved’
In Alzheimer's disease clinical trials, ‘time saved’ refers to the estimated amount of time a treatment delays the progression of the disease, allowing patients to maintain functionality and independence longer. This approach provides a clinically meaningful measure, as it directly relates to the impact on a patient's daily life.4,5 Additionally, blarcamesine exhibited a favorable safety profile with no treatment-related deaths.
In the Phase IIb/III oral blarcamesine early Alzheimer’s disease trial, oral blarcamesine resulted in 77.4 weeks (approximately 17.8 months) of time saved in the ITT population compared to the ADNI control group. This measure provides a meaningful clinical perspective, emphasizing the real-world impact of treatment on patients’ daily lives and highlighting the potential for long-term therapeutic benefit.
Restoring impaired autophagy
The clinical trial data on blarcamesine also confirmed the upstream mechanism of blarcamesine, restoring impaired autophagy as an early event, preceding amyloid-beta and tau.
The mechanistic confirmation that blarcamesine restores impaired autophagy through SIGMAR1 activation by acting upstream of amyloid and tau pathologies at the molecular level was previously established both in vitro and in vivo. Specifically, studies demonstrated enhanced autophagic flux in human cells and in C. elegans as well as increased proteostasis capacity, ultimately ameliorating paralysis caused by protein aggregation in C. elegans.6
“We remain excited about these enhanced clinically meaningful improvements and accompanied by blarcamesine’s favorable safety profile,” said Juan Carlos Lopez-Talavera, MD, PhD, Head of Research and Development of Anavex. “Convenient once-daily oral dosing of blarcamesine may allow us to offer a scalable and patient friendly oral pill administration option to patients with early Alzheimer’s.”
“We are inspired to advancing science across this devastating chronic disease. Alzheimer’s disease, like other chronic progressive diseases, requires a long-term therapeutic strategy. Blarcamesine with its convenient once daily oral dosing may lead to greater clinical benefit as evidenced by the significant improvement versus ADNI control group.,” said Christopher U Missling, PhD, President and Chief Executive Officer of Anavex. “Additionally, this could help reduce crucial barriers within the currently complex healthcare ecosystem for Alzheimer's disease and potentially provide broader access to a diverse population with early Alzheimer's disease.”
Anavex plans to publish and present this new data at international Alzheimer’s disease conferences.
This release discusses investigational uses of an agent in development and is not intended to convey conclusions about efficacy or safety. There is no guarantee that any investigational uses of such product will successfully complete clinical development or gain health authority approval.
About Anavex Life Sciences Corp.
Anavex Life Sciences Corp. (Nasdaq: AVXL) is a publicly traded biopharmaceutical company dedicated to the development of novel therapeutics for the treatment of neurodegenerative, neurodevelopmental, and neuropsychiatric disorders, including Alzheimer's disease, Parkinson's disease, schizophrenia, Rett syndrome, and other central nervous system (CNS) diseases, pain, and various types of cancer. Anavex's lead drug candidate, ANAVEX®2-73 (blarcamesine), has successfully completed a Phase 2a and a Phase 2b/3 clinical trial for Alzheimer's disease, a Phase 2 proof-of-concept study in Parkinson's disease dementia, and both a Phase 2 and a Phase 3 study in adult patients and one Phase 2/3 study in pediatric patients with Rett syndrome. ANAVEX®2-73 is an orally available drug candidate designed to restore cellular homeostasis by targeting SIGMAR1 and muscarinic receptors. Preclinical studies demonstrated its potential to halt and/or reverse the course of Alzheimer's disease. ANAVEX®2-73 also exhibited anticonvulsant, anti-amnesic, neuroprotective, and anti-depressant properties in animal models, indicating its potential to treat additional CNS disorders, including epilepsy. The Michael J. Fox Foundation for Parkinson's Research previously awarded Anavex a research grant, which fully funded a preclinical study to develop ANAVEX®2-73 for the treatment of Parkinson's disease. We believe that ANAVEX®3-71, which targets SIGMAR1 and M1 muscarinic receptors, is a promising clinical stage drug candidate demonstrating disease-modifying activity against the major hallmarks of Alzheimer's disease in transgenic (3xTg-AD) mice, including cognitive deficits, amyloid, and tau pathologies. In preclinical trials, ANAVEX®3-71 has shown beneficial effects on mitochondrial dysfunction and neuroinflammation. Further information is available at www.anavex.com. You can also connect with the Company on Twitter, Facebook, Instagram, and LinkedIn.
Forward-Looking Statements
Statements in this press release that are not strictly historical in nature are forward-looking statements. These statements are only predictions based on current information and expectations and involve a number of risks and uncertainties. Actual events or results may differ materially from those projected in any of such statements due to various factors, including the risks set forth in the Company’s most recent Annual Report on Form 10-K filed with the SEC. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement and Anavex Life Sciences Corp. undertakes no obligation to revise or update this press release to reflect events or circumstances after the date hereof.
For Further Information:
Anavex Life Sciences Corp.
Research & Business Development
Toll-free: 1-844-689-3939
Email: info@anavex.com
Investors:
Andrew J. Barwicki
Investor Relations
Tel: 516-662-9461
Email: andrew@barwicki.com
1 Alzheimer’s Disease Neuroimaging Initiative (ADNI) is a clinical research project launched by NIH in 2004 to develop methods to predict the onset and progression of Alzheimer’s disease and to confirm the effectiveness of treatments. The project involves a multi-year longitudinal observation targeting healthy elderly individuals as well as patients with mild cognitive impairment (MCI) and early stages of Alzheimer’s disease.
2 Observed raw data was used. Scheduled visits were [OLE Week 0 = Combined Week 48], [OLE Week 48 = Combined Week 96], [OLE Week 96 = Combined Week 144]; Combined = DB (double-blind) + OLE (open-label-extension) trials. Up to 144 weeks ADNI control group data was available.
3 ADAS-Cog13 scores LS mean difference between the treatment groups being larger than 2 points are considered clinically meaningful improvements: Muir RT, Hill MD, Black SE, Smith EE. Minimal clinically important difference in Alzheimer's disease: Rapid review. Alzheimers Dement. 2024;20(5):3352-3363.
4 Petersen, R C et al. “Expectations and clinical meaningfulness of randomized controlled trials.” Alzheimer's & dementia: the journal of the Alzheimer's Association vol. 19,6 (2023): 2730-2736.
5 Dickson, S P et al. “"Time Saved" Calculations to Improve Decision-Making in Progressive Disease Studies.” The journal of prevention of Alzheimer's disease. vol. 11,3 (2024): 529-536.
6 Christ, M G et al. “Sigma-1 Receptor Activation Induces Autophagy and Increases Proteostasis Capacity In Vitro and In Vivo.” Cells vol. 8,3 211. 2 Mar. 2019.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/f19a8c2c-6ef7-43f8-b9dd-771d15db5d15








