BriaCell Images Confirm Robust Anti-Tumor Activity in Patient with “Eye-Bulging” Metastatic Breast Cancer
- Significant reduction of metastatic breast cancer tumor behind-the-eye after only 3 cycles
- Powerful anti-tumor response associated with reduction in proptosis (eye-bulging) and reduced ocular pain
- Heavily pre-treated patient had failed 7 prior regimens including antibody-drug conjugate therapy and remains on BriaCell treatment
PHILADELPHIA and VANCOUVER, British Columbia, Jan. 04, 2024 (GLOBE NEWSWIRE) -- BriaCell Therapeutics Corp. (Nasdaq: BCTX, BCTXW) (TSX: BCT) (“BriaCell” or the “Company”), a clinical-stage biotechnology company that develops novel immunotherapies to transform cancer care, is pleased to release transformational images of the recently reported remarkable responder in the Phase 2 study of BriaCell’s Bria-IMT™ combination regimen. The patient had metastatic breast cancer behind her eye, causing proptosis (eye-bulging) and significant pain that were both markedly reduced with BriaCell’s treatment.
“We are extremely excited to report significant tumor reduction in this very difficult to treat patient who had failed 7 prior regimens including treatment with Enhertu®, an antibody-drug conjugate, highlighting the robust anti-tumor activity of the Bria-IMT™ regimen in difficult to reach tumors such as those in the eye orbit. We observed significant tumor reduction along with significant eye pain reduction after only 3 cycles of treatment with the Bria-IMT™ combination regimen. The Bria-IMT™ regimen has been very well tolerated and the patient remains on treatment,” stated Dr. William V. Williams, BriaCell’s President and CEO. “We look forward to sharing additional data in the coming months.”
The following figure shows magnetic resonance imaging (MRI) of the orbital tumor. The top left MRI image shows the tumor in the right orbit behind the eye with the eye not being visible pre-treatment. After treatment with the Bria-IMT™ regimen, the eye becomes visible (top right image) as it has regained its normal position. In the lower images, the dashed line represents normal margin of eye position with resolution of proptosis post treatment (small arrows) with the Bria-IMT™ regimen. Reduction in tumor is represented by large arrows.
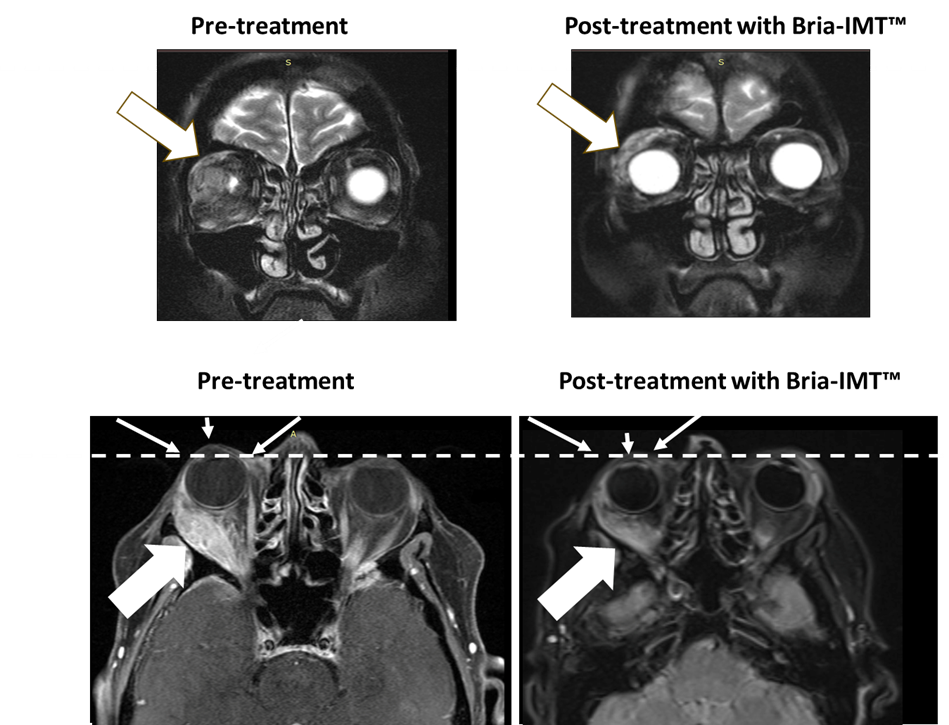
BriaCell had previously reported a similar case of a remarkable response with resolution of an eye-bulging orbital tumor. That particular patient had received (and failed) 12 regimens with 16 agents (incl. 13 chemotherapies) prior to BriaCell’s combination therapy, again adding to the remarkable nature of her response. These two patient responses are included in BriaCell’s recently reported
“Today’s reported MRI imaging confirms the clinical response seen and supports the further development of our Bria-IMT™ regimen. Women with metastatic breast cancer continue to have poor survival despite recently approved therapies and further development of novel treatments remains an area of high unmet medical need,” stated Dr. Giuseppe Del Priore, BriaCell’s Chief Medical Officer.
The Bria-IMT™ combination regimen is currently undergoing a pivotal Phase 3 study in advanced breast cancer.
About BriaCell Therapeutics Corp.
BriaCell is a clinical-stage biotechnology company that develops novel immunotherapies to transform cancer care. More information is available at https://briacell.com/.
Safe Harbor
This press release contains “forward-looking statements” that are subject to substantial risks and uncertainties. All statements, other than statements of historical fact, contained in this press release are forward-looking statements. Forward-looking statements contained in this press release may be identified by the use of words such as “anticipate,” “believe,” “contemplate,” “could,” “estimate,” “expect,” “intend,” “seek,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “target,” “aim,” “should,” “will,” “would,” or the negative of these words or other similar expressions, although not all forward-looking statements contain these words. Forward-looking statements, including those about: the presentation of additional data in the coming months are subject to inherent uncertainties, risks, and assumptions that are difficult to predict. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate. These and other risks and uncertainties are described more fully under the heading “Risks and Uncertainties” in the Company's most recent Management’s Discussion and Analysis, under the heading "Risk Factors" in the Company's most recent Annual Information Form, and under “Risks and Uncertainties” in the Company's other filings with the Canadian securities regulatory authorities and the U.S. Securities and Exchange Commission, all of which are available under the Company's profiles on SEDAR+ at www.sedarplus.ca and on EDGAR at www.sec.gov. Forward-looking statements contained in this announcement are made as of this date, and BriaCell Therapeutics Corp. undertakes no duty to update such information except as required under applicable law.
Neither the Toronto Stock Exchange nor its Regulation Services Provider (as that term is defined in the policies of the Toronto Stock Exchange) accepts responsibility for the adequacy or accuracy of this release.
Contact Information
Company Contact:
William V. Williams, MD
President & CEO
1-888-485-6340
info@briacell.com
Media Relations:
Jules Abraham
Director of Public Relations
CORE IR
917-885-7378
julesa@coreir.com
Investor Relations Contact:
CORE IR
investors@briacell.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/eecec66c-76aa-4386-be71-6489abad159e








