BD Receives FDA 510(k) Clearance for EnCor EnCompass™ Breast Biopsy and Tissue Removal System
Rhea-AI Summary
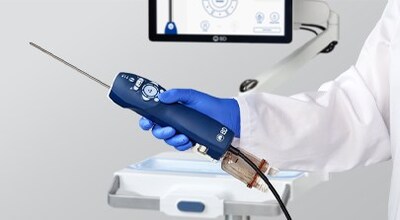
BD (NYSE: BDX) received FDA 510(k) clearance for the EnCor EnCompass™ Breast Biopsy and Tissue Removal System on January 15, 2026. The multi-modality biopsy system is designed to work across breast imaging platforms, offering adjustable vacuum strengths, a variable sample notch, 360° sampling, echogenic cutting cannula, illuminated sample container, and probe choices of 12G, 10G, and 7G. The system is expected to enter the market in early 2026, expanding BD's breast health portfolio to support earlier detection and diagnosis.
Positive
- FDA 510(k) clearance received on Jan 15, 2026
- Market entry planned for early 2026
- Designed for multi-modality use across imaging platforms
- 360° sampling capability for comprehensive lesion access
- Offers 12G, 10G, and 7G probe options
Negative
- None.
News Market Reaction
On the day this news was published, BDX gained 0.45%, reflecting a mild positive market reaction.
Data tracked by StockTitan Argus on the day of publication.
Key Figures
Market Reality Check
Peers on Argus
BDX was up 2.93% while key peers ALC (0.6%), RMD (2.31%), WST (0.75%), BAX (1.17%) and HOLX (0.35%) also gained modestly, but no peers appeared in the momentum scanner, suggesting a more stock-specific move around BDX.
Historical Context
| Date | Event | Sentiment | Move | Catalyst |
|---|---|---|---|---|
| Jan 13 | Capacity investment | Positive | -0.6% | $110M Neopak syringe expansion and U.S. manufacturing investment plan. |
| Jan 12 | Shareholder meeting | Neutral | -0.6% | Announcement of fully virtual 2026 annual shareholders meeting logistics. |
| Jan 05 | Clinical milestone | Positive | +2.1% | First Phasix hernia prevention case in Greece and >85% PREVENT enrollment. |
| Dec 18 | Research collaboration | Positive | -0.3% | Collaboration with Penn on 1,000-patient immune profiling via flow cytometry. |
| Dec 15 | Product expansion | Positive | -1.9% | IVDR certification for new VIASURE assays on BD MAX system in Europe. |
Recent history shows several positive strategic and clinical updates often met with flat to slightly negative next-day moves, with only one clear positive alignment.
Over the last month, BD reported multiple developments across manufacturing, clinical programs and collaborations. A $110 million U.S. syringe investment and related $2.5 billion five-year manufacturing pledge preceded a -0.6% move. A virtual 2026 shareholder meeting also saw a -0.6% reaction. Clinical progress with Phasix™ and the PREVENT trial (> 85% enrolled, results targeted for 2026) aligned with a 2.06% gain. Collaborations in immunotherapy and IVDR-certified assays in Europe drew small negative reactions. Today’s FDA 510(k) clearance adds another product-focused milestone to this stream of updates.
Market Pulse Summary
This announcement highlights FDA 510(k) clearance for BD’s EnCor EnCompass breast biopsy and tissue removal system, with commercial entry targeted for early 2026. It adds to a series of recent product, clinical and collaboration milestones across BD’s portfolio. Investors may focus on how this multi-modality platform integrates into the existing breast health franchise, adoption across imaging modalities, and execution against previously disclosed manufacturing and clinical initiatives when assessing the longer-term impact.
Key Terms
510(k) clearance regulatory
multi-modality medical
breast biopsy medical
echogenic medical
cannula medical
AI-generated analysis. Not financial advice.
State-of-the-art, multi-modality breast biopsy system slated to be in the market in early 2026
FRANKLIN LAKES, N.J., Jan. 15, 2026 /PRNewswire/ -- BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today announced that the
"This milestone for our new breast biopsy system marks a meaningful advancement in breast health, playing a critical role in aiding the early detection and diagnosis of breast disease," said Rima Alameddine, worldwide president, Peripheral Intervention at BD. "This innovation underscores our commitment to partnering with clinical leaders to deliver patient-centered solutions. Guided by our vision to transform breast health, we remain focused on developing technologies that empower providers and inspire confidence in care."
The EnCor EnCompass™ Biopsy System is designed to streamline the breast biopsy experience by enabling clinicians to perform procedures across a range of breast imaging platforms using one integrated system. The system, which is expected to enter the market in early 2026, offers a combination of advanced features and user-focused design to support procedural efficiency.
"The FDA clearance of the EnCor EnCompass™ Biopsy System demonstrates our ongoing focus on addressing the evolving needs of clinicians and patients in breast health," said Stacie Watson, vice president and general manager of the Oncology Platform at BD Interventional–Peripheral Intervention. "This multi-modality platform is engineered to provide flexibility, control, and ease of use, with features designed to support both clinician confidence and the patient experience."
Key features of the EnCor EnCompass™ Biopsy System include:
- Multi-modality use to support procedures performed across breast imaging platforms
- High and low vacuum strengths and a variable sample notch that can be adjusted during the procedure
- 360° sampling capability to access lesions located throughout the breast
- Features to enhance visualization, including an echogenic cutting cannula and illuminated sample container
- Choice of 12G, 10G, and 7G probes to accommodate different lesion types and locations
"Our goal is always to provide the best possible care for patients while maintaining efficiency, accuracy, and safety," said Dr. Shadi Aminololama-Shakeri, M.D., Chief of Breast Radiology at UC Davis. "The EnCor EnCompass™ Biopsy System combines multi-modality capability and enhanced control into one platform that supports intraprocedural customization and helps streamline the biopsy process."
The clearance of the EnCor EnCompass™ Biopsy System expands BD's breast health portfolio and reflects the Company's ongoing commitment to advancing technologies that support early detection and diagnosis.
About BD
BD is one of the largest global medical technology companies in the world and is advancing the world of health™ by improving medical discovery, diagnostics and the delivery of care. The company supports the heroes on the frontlines of health care by developing innovative technology, services and solutions that help advance both clinical therapy for patients and clinical process for health care providers. BD and its more than 70,000 employees have a passion and commitment to help enhance the safety and efficiency of clinicians' care delivery process, enable laboratory scientists to accurately detect disease and advance researchers' capabilities to develop the next generation of diagnostics and therapeutics. BD has a presence in virtually every country and partners with organizations around the world to address some of the most challenging global health issues. By working in close collaboration with customers, BD can help enhance outcomes, lower costs, increase efficiency, improve safety and expand access to health care. For more information on BD, please visit bd.com or connect with us on LinkedIn at www.linkedin.com/company/bd1/, X (formerly Twitter) at @BDandCo or Instagram at @becton_dickinson.
Contacts: | |
Media: | Investors: |
Fallon McLoughlin | Adam Reiffe |
Director, Public Relations | Vice President, Investor Relations |
201.258.0361 | 201.847.6927 |
Indications for Use: The EnCor EnCompass™ Breast Biopsy and Tissue Removal System is indicated to acquire breast tissue for histologic examination with partial or complete removal of the abnormality.
Contraindications: 1.) This device is not intended for use except as indicated. 2.) The EnCor EnCompass™ Breast Biopsy and Tissue Removal System is contraindicated for those patients where, in the physician's judgment, there is an increased risk of complications associated with percutaneous removal of tissue samples.
Warnings: Attention: Histologic findings require correlation with imaging and clinical findings to determine concordance. If there is discordance between imaging, clinical findings, and/or pathology, further histological evaluation and potential, additional diagnostic or excisional procedures are still needed. Whenever breast tissue is removed, histological evaluation of the tissue should be performed per standard of care. Clinical and imaging surveillance should be conducted in accordance with established clinical practice guidelines and institutional protocols.
Potential Complications: Potential complications include, but are not limited to, hematoma, hemorrhage, hemothorax, pneumothorax, surgical intervention, infection, adjacent tissue injury, pain, allergic reaction, and tissue adherence to the biopsy probe during removal from the breast.
For more information, please consult the product labels and inserts for any indication, contraindications, hazards, precautions
and directions for use.
BD, the BD Logo, Advancing the world of health and EnCor EnCompass are trademarks of Becton, Dickinson and Company or its affiliates. © 2026 BD. All Rights Reserved. BD-165500
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/bd-receives-fda-510k-clearance-for-encor-encompass-breast-biopsy-and-tissue-removal-system-302661681.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/bd-receives-fda-510k-clearance-for-encor-encompass-breast-biopsy-and-tissue-removal-system-302661681.html
SOURCE BD (Becton, Dickinson and Company)