Can-Fite: Scientific Article Published by KOL Presents Namodenoson as a Promising Drug Candidate to Treat Advanced Liver Cancer and MASH
A pivotal Phase III trial for advanced liver cancer, approved by FDA & EMA and a MASH Phase IIb are enrolling patients
PETACH TIKVA,
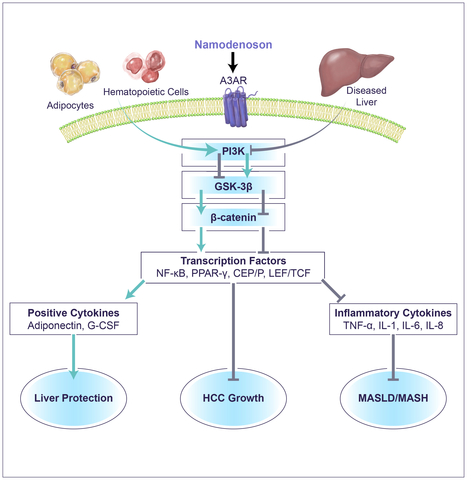
Namodenoson mechanism of action (Graphic: Business Wire)
The article’s first author, Dr. Ohad Etzion, is a renowned key opinion leader (KOL) in the Hepatology field and is the engine for some of the novel drugs under development for the treatment of MASH and additional liver diseases. Dr. Etzion is the Head, Department of Gastroenterology and Liver Diseases, at Soroka University Medical Center,
The Biomedicines article presents the pre-clinical and clinical activity of Namodenoson and the mechanism of action through which the drug induces the anti-cancer activity and at the same time the liver protective effects. This dual activity enables the drug to have positive effects in both liver cancer and MASH.
Currently, Namodenoson is being evaluated in LiverationTM, a pivotal Phase III study for advanced liver cancer that has been approved by both the
“We are very much encouraged by the clinical data from the Phase II study in HCC and the Phase IIa study in MASH. The drug mechanism of action which is presented in the manuscript and entails inflammatory cytokine inhibition together with the stimulation of positive cytokines, position Namodenoson as a promising safe drug,” stated Dr. Etzion.
About Namodenoson
Namodenoson is a small orally bioavailable drug that binds with high affinity and selectivity to the A3 adenosine receptor (A3AR). Namodenoson was evaluated in Phase II trials for two indications, as a second line treatment for hepatocellular carcinoma, and as a treatment for non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). A3AR is highly expressed in diseased cells whereas low expression is found in normal cells. This differential effect accounts for the excellent safety profile of the drug.
About Can-Fite BioPharma Ltd.
Can-Fite BioPharma Ltd. (NYSE American: CANF) (TASE: CANF) is an advanced clinical stage drug development Company with a platform technology that is designed to address multi-billion dollar markets in the treatment of cancer, liver, and inflammatory disease. The Company's lead drug candidate, Piclidenoson recently reported topline results in a Phase III trial for psoriasis and is expected to commence a pivotal Phase III. Can-Fite's cancer and liver drug, Namodenoson, is being evaluated in a Phase IIb trial for the treatment metabolic dysfunction-associated steatohepatitis (MASH), a Phase III pivotal trial for hepatocellular carcinoma (HCC), and the Company is planning a Phase IIa study in pancreatic cancer. Namodenoson has been granted Orphan Drug Designation in the
Forward-Looking Statements
This press release may contain forward-looking statements, about Can-Fite’s expectations, beliefs or intentions regarding, among other things, its product development efforts, business, financial condition, results of operations, strategies or prospects. All statements in this communication, other than those relating to historical facts, are “forward looking statements”. Forward-looking statements can be identified by the use of forward-looking words such as “believe,” “expect,” “intend,” “plan,” “may,” “should” or “anticipate” or their negatives or other variations of these words or other comparable words or by the fact that these statements do not relate strictly to historical or current matters. Forward-looking statements relate to anticipated or expected events, activities, trends or results as of the date they are made. Because forward-looking statements relate to matters that have not yet occurred, these statements are inherently subject to known and unknown risks, uncertainties and other factors that may cause Can-Fite’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Important factors that could cause actual results, performance or achievements to differ materially from those anticipated in these forward-looking statements include, among other things, our history of losses and needs for additional capital to fund our operations and our inability to obtain additional capital on acceptable terms, or at all; uncertainties of cash flows and inability to meet working capital needs; the initiation, timing, progress and results of our preclinical studies, clinical trials and other product candidate development efforts; our ability to advance our product candidates into clinical trials or to successfully complete our preclinical studies or clinical trials; our receipt of regulatory approvals for our product candidates, and the timing of other regulatory filings and approvals; the clinical development, commercialization and market acceptance of our product candidates; our ability to establish and maintain strategic partnerships and other corporate collaborations; the implementation of our business model and strategic plans for our business and product candidates; the scope of protection we are able to establish and maintain for intellectual property rights covering our product candidates and our ability to operate our business without infringing the intellectual property rights of others; competitive companies, technologies and our industry; risks related to any resurgence of the COVID-19 pandemic and the war between
View source version on businesswire.com: https://www.businesswire.com/news/home/20240415044511/en/
Can-Fite BioPharma
Motti Farbstein
info@canfite.com
+972-3-9241114
Source: Can-Fite BioPharma Ltd.







