CollPlant's rhCollagen-Based BioInk Demonstrates Strong Performance Compared to Matrigel® in Study Conducted by Technion - Israel Institute of Technology
Rhea-AI Summary
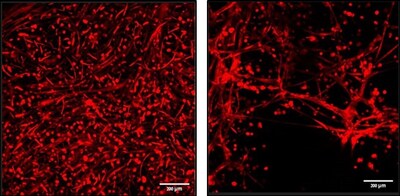
CollPlant (Nasdaq: CLGN) announced that a head-to-head study by Technion's Levenberg Lab found its rhCollagen-based bioink Collink.3D™ outperformed Matrigel® on key lab metrics. Collink.3D™ reportedly showed enhanced mechanical strength, elasticity, and stability, supporting organized and long-lasting tissue formation in a reproducible, animal-free format.
The release highlights potential application in tissue engineering, regenerative medicine, and drug discovery and notes the global basement membrane matrix market was ~$96M in 2024, projected to reach $201M by 2031 (CAGR 11.2%).
Positive
- None.
Negative
- None.
News Market Reaction – CLGN
On the day this news was published, CLGN gained 7.33%, reflecting a notable positive market reaction. Argus tracked a peak move of +9.0% during that session. Our momentum scanner triggered 4 alerts that day, indicating moderate trading interest and price volatility. This price movement added approximately $2M to the company's valuation, bringing the market cap to $34M at that time.
Data tracked by StockTitan Argus on the day of publication.
- A head-to-head study by
Israel's Technion found that CollPlant's rhCollagen-based bioink, Collink.3D™, outperformed Matrigel®, a leading extracellular matrix, in supporting structured tissue formation. - The findings suggest Collink.3D™ could offer a consistent, tunable, and animal-free alternative for advanced tissue engineering and research applications.
- Extracellular matrices are key tools in drug discovery, regenerative medicine, and tissue modeling. CollPlant's plant-based technology provides a sustainable and ethical approach without compromising performance.
REHOVOT,
Matrigel®, developed and marketed by Corning, has been one of the most widely used extracellular matrices for more than three decades. Derived from the Engelbreth-Holm-Swarm (EHS) mouse sarcoma, it contains a rich combination of basement membrane proteins such as laminin, collagen IV, heparan sulfate proteoglycans, and entactin/nidogen, as well as growth factors that make it a cornerstone for 3D cell culture, disease modeling, and drug discovery.
In the Technion study, Collink.3D™ demonstrated enhanced mechanical strength, elasticity, and stability, providing a reproducible environment that supports organized and long-lasting tissue formation. These results suggest that Collink.3D™ may serve as a next-generation, animal-free extracellular matrix, offering a complementary approach to traditional matrices such as Matrigel®.
The global market for basement membrane matrices, including Matrigel and similar products, was valued at approximately
Yehiel Tal, Chief Executive Officer of CollPlant, commented: "We are very pleased with the outcome of this study by the Technion. The results reinforce Collink.3D™ as a biologically supportive and durable bioink that promotes cell organization and tissue development while maintaining structural integrity over time. We believe Collink.3D™ represents a strong, animal-free alternative for tissue engineering, regenerative medicine, and drug discovery applications."
About Collink.3D ™
Collink.3D™ is a recombinant human type I collagen methacrylamide bioink, produced using genetically engineered plants and free of any animal-derived substances or reagents. It is the first and only plant-based human-collagen bioink platform, designed to closely mimic the native properties of human tissues and organs.
Collink.3D™ enables scalable and reproducible biofabrication of 3D tissue and organ models, including disease models for drug screening and therapeutic research, as well as scaffolds, tissues, and organs intended for implantation. It supports the creation of complex 3D architectures with defined geometric, physical, and biological properties - offering a sustainable and high-performance solution for the rapidly growing field of regenerative medicine.
About CollPlant
CollPlant is a regenerative and aesthetic medicine company ushering in a new era of medical solutions with a focus on 3D bioprinting of tissues and organs, tissue repair and medical aesthetics. The Company's products are based on its rhCollagen (recombinant human collagen) produced with CollPlant's proprietary plant-based genetic engineering technology. These products address indications within the diverse fields of tissue repair, aesthetics, and organ manufacturing.
In 2021, CollPlant entered into a development and global commercialization agreement for dermal and soft tissue fillers with Allergan, an AbbVie company, the global leader in the dermal filler market.
For more information about CollPlant, visit http://www.collplant.com.
Forward-Looking Statements
This press release may include forward-looking statements. Forward-looking statements may include, but are not limited to, statements relating to CollPlant's objectives plans and strategies, as well as statements, other than historical facts, that address activities, events or developments that CollPlant intends, expects, projects, believes or anticipates will or may occur in the future. These statements are often characterized by terminology such as "believes," "hopes," "may," "anticipates," "should," "intends," "plans," "will," "expects," "estimates," "projects," "positioned," "strategy" and similar expressions and are based on assumptions and assessments made in light of management's experience and perception of historical trends, current conditions, expected future developments and other factors believed to be appropriate.
Forward-looking statements are not guarantees of future performance and are subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied in such statements. Many factors could cause CollPlant's actual activities or results to differ materially from the activities and results anticipated in forward-looking statements, including, but not limited to, the following: the Company's history of significant losses, its need to raise additional capital and its inability to obtain additional capital on acceptable terms, or at all; the Company's expectations regarding the costs and timing of commencing and/or concluding pre-clinical and clinical trials with respect to breast implants, tissues and organs which are based on its rhCollagen based BioInk and other products for medical aesthetics, and specifically the Company's ability to initiate its next large-animal study for its breast implants in a timely manner, or at all; the Company's or it strategic partners' ability to obtain favorable pre-clinical and clinical trial results; regulatory action with respect to rhCollagen based bioink and medical aesthetics products or product candidates including, but not limited to acceptance of an application for marketing authorization review and approval of such application, and, if approved, the scope of the approved indication and labeling; commercial success and market acceptance of the Company's rhCollagen based products, in 3D bioprinting and medical aesthetics; the Company's ability to establish sales and marketing capabilities or enter into agreements with third parties and its reliance on third party distributors and resellers; the Company's ability to establish and maintain strategic partnerships and other corporate collaborations, including its partnership with AbbVie and its ability to continue to receive milestone and royalties payments under the AbbVie agreement; the Company's reliance on third parties to conduct some or all aspects of its product development and manufacturing; the scope of protection the Company is able to establish and maintain for intellectual property rights and the Company's ability to operate its business without infringing the intellectual property rights of others; current or future unfavorable economic and market conditions and adverse developments with respect to financial institutions and associated liquidity risk; the impact of competition and new technologies; general market, political, and economic conditions in the countries in which the Company operates, including, with respect to the ongoing war in
Contacts
CollPlant:
Eran Rotem
Deputy CEO & CFO
+ 972-73-2325600
Eran@collplant.com
Investors:
LifeSci Advisors
Dan Ferry
daniel@lifesciadvisors.com
Photo - https://mma.prnewswire.com/media/2800257/CollPlant_Photo.jpg
Logo - https://mma.prnewswire.com/media/2217353/CollPlant_Logo.jpg

![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/collplants-rhcollagen-based-bioink-demonstrates-strong-performance-compared-to-matrigel-in-study-conducted-by-technion--israel-institute-of-technology-302588881.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/collplants-rhcollagen-based-bioink-demonstrates-strong-performance-compared-to-matrigel-in-study-conducted-by-technion--israel-institute-of-technology-302588881.html
SOURCE CollPlant
FAQ
What did Technion find about CollPlant's Collink.3D™ versus Matrigel in the Oct 20, 2025 study for CLGN?
How could Collink.3D™ affect tissue engineering and drug discovery for CLGN?
Does the Oct 20, 2025 announcement for CLGN include commercial deals or revenue figures?
What market size did CollPlant cite for basement membrane matrices in the Oct 20, 2025 release for CLGN?
Will the Technion study change CollPlant's (CLGN) regulatory or approval pathway?
What are the next steps investors should look for after CollPlant's Oct 20, 2025 study release for CLGN?