ClearPoint Neuro Announces FDA Clearance and First-in-Human Cases Performed with the New 2.2 Software Version and the Integrated Maestro Brain Model
Clinical Validation of the Brain Model Published Online in the Journal NeuroImage
SOLANA BEACH, Calif., Feb. 21, 2024 (GLOBE NEWSWIRE) -- ClearPoint Neuro, Inc. (Nasdaq: CLPT) (the “Company”), a global device, cell, and gene therapy-enabling company offering precise navigation to the brain and spine, today announced FDA Clearance and first-in-human cases using the ClearPoint 2.2 Software with integrated Maestro Brain Modeling, and also the publication of a key validation study for its ClearPoint Maestro® Brain Model in the peer-reviewed journal NeuroImage.1
“Leadership and innovation in any medical field requires not only practical product development to deliver value for clinicians, but also robust validation and peer review to show how and why those products work,” commented Joe Burnett, President and CEO at ClearPoint Neuro. “The Maestro Brain Model, and its integration into the recently FDA cleared ClearPoint 2.2 navigation software, is our latest example of just that. The ClearPoint system can now offer fast, peri-procedural segmentation of the cortical structures of the brain to identify both targets and safety zones for cell and gene therapy delivery, laser ablation, biopsy and deep brain stimulation. At the same time, we are pleased to announce that one of many planned validation studies has been published online in the journal NeuroImage to give surgeons confidence in the performance and accuracy of the Maestro Tool. The first clinical cases using ClearPoint 2.2 were completed successfully this quarter, and we expect full market release in the second half of this year.”
MRI-guided neuro interventions require rapid, accurate, and reproducible segmentation of anatomical brain structures for identification of targets during surgical procedures. This manuscript introduces the methodology for shape-constrained deformable brain segmentation behind Maestro, describes the validation performed for its FDA clearance, and presents a comparison with manual expert segmentation and FreeSurfer, an open-source segmentation software. Quantitative analysis indicates superior performance compared to both manual expert segmentation and FreeSurfer. The shape-constrained methodology results in accurate and highly reproducible segmentation. Furthermore, inherent point based-correspondence provides consistent target identification ideal for MRI-guided neuro interventions. A link to the open source publication can be found here.
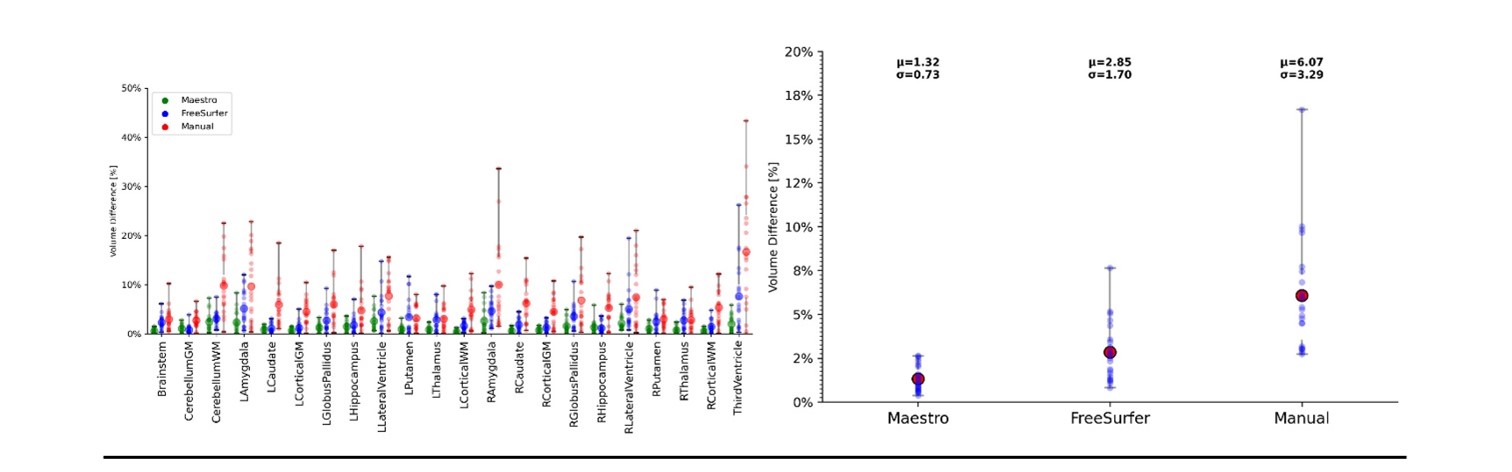
Reproducibility error of Maestro (green), FreeSurfer 7.2 (blue) and manual segmentation (red) for common brain structures (left), and average over all structures (right). Bars represent range of measured relative volume difference.
About ClearPoint Neuro
ClearPoint Neuro is a device, cell, and gene therapy-enabling company offering precise navigation to the brain and spine. The Company uniquely provides both established clinical products as well as preclinical development services for controlled drug and device delivery. The Company’s flagship product, the ClearPoint Neuro Navigation System, has FDA clearance and is CE-marked. ClearPoint Neuro is engaged with healthcare and research centers in North America, Europe, Asia, and South America. The Company is also partnered with the most innovative pharmaceutical/biotech companies, academic centers, and contract research organizations, providing solutions for direct CNS delivery of therapeutics in pre-clinical studies and clinical trials worldwide. To date, thousands of procedures have been performed and supported by the Company’s field-based clinical specialist team, which offers support and services to our customers and partners worldwide. For more information, please visit www.clearpointneuro.com.
Forward-Looking Statements
This press release contains forward-looking statements within the context of the federal securities laws, which may include the Company’s expectation for the future market of its products and services, and other performance and results. These forward-looking statements are based on management’s current expectations and are subject to the risks inherent in the business, which may cause the Company's actual results to differ materially from those expressed in or implied by forward-looking statements. Particular uncertainties and risks include those relating to: global and political instability, supply chain disruptions, labor shortages, and macroeconomic and inflationary conditions; future revenue from sales of the Company’s products and services; the Company’s ability to market, commercialize and achieve broader market acceptance for new products and services offered by the Company; the ability of our biologics and drug delivery partners to achieve commercial success, including their use of the Company’s products and services in their delivery of therapies; the Company’s expectations, projections and estimates regarding expenses, future revenue, capital requirements, and the availability of and the need for additional financing; the Company’s ability to obtain additional funding to support its research and development programs; the ability of the Company to manage the growth of its business; the Company’s ability to attract and retain its key employees; and risks inherent in the research, development, and regulatory approval of new products. More detailed information on these and additional factors that could affect the Company’s actual results are described in the “Risk Factors” section of the Company’s Annual Report on Form 10-K for the year ended December 31, 2022, and the Company’s Quarterly Report on Form 10-Q for the three months ended September 30, 2023, both of which have been filed with the Securities and Exchange Commission, and the Company’s Annual Report on Form 10-K for the year ended December 31, 2023, which the Company intends to file with the Securities and Exchange Commission on or before March 31, 2024. The Company does not assume any obligation to update these forward-looking statements.
_____________________
1 Zagorchev L, Hyde DE, Li C, et al. Shape-constrained deformable brain segmentation: Methods and quantitative validation. Neuroimage. Published online February 16, 2024. doi:10.1016/j.neuroimage.2024.120542
Photos accompanying this announcement are available at https://www.globenewswire.com/NewsRoom/AttachmentNg/088f3809-a329-47e4-bfc0-21655bb9e1b2
https://www.globenewswire.com/NewsRoom/AttachmentNg/312932db-1574-4a9c-9bc4-5218308a2cee