CLINUVEL recruits 200 patients in Phase III vitiligo trial CUV105
Rhea-AI Summary
CLINUVEL (CLVLY) has achieved its recruitment target of 200 patients for its Phase III trial (CUV105) evaluating SCENESSE® (afamelanotide 16mg) in vitiligo patients. The study, conducted across 37 sites in three continents with 57% of patients from the US, focuses on adolescent and adult patients with darker skin types (Fitzpatrick III-VI).
The trial compares SCENESSE® administered every three weeks plus narrowband ultraviolet B (NB-UVB) phototherapy against NB-UVB monotherapy alone over a 20-week treatment period with a 6-month follow-up. Initial results are expected in 2H 2026. Early clinical observations from five case studies show promising repigmentation results, including a notable case of a skin type V patient with 20-year vitiligo history showing improvement in arm and leg lesions.
Positive
- Clinical observations from five case studies show positive repigmentation results
- Treatment protocol includes option for NB-UVB monotherapy patients to receive SCENESSE® after follow-up period
- Early case studies demonstrate repigmentation as quick as four weeks after treatment initiation
- Building North American distribution network among dermatologists ahead of potential market entry
Negative
- Long timeline to results - first data not expected until second half of 2026
- Extended treatment protocol of 20 weeks plus 6-month follow-up period required
- Will require a second large trial (CUV107) for evaluation
First results of SCENESSE® study expected in 2H 2026
Executive summary
- Recruitment target of 200 vitiligo patients (Fitzpatrick skin type III-VI) in CUV105 study achieved
- Randomised trial, 20-week treatment protocol plus 6-month follow up
- Study sites in North America, Africa and Europe
- First clinical observations with afamelanotide positive
MELBOURNE, Australia, May 07, 2025 (GLOBE NEWSWIRE) -- CLINUVEL has met its recruitment target in its phase III trial (CUV105) of SCENESSE® (afamelanotide 16mg) in vitiligo, with more than 200 patients enrolled. The last patient to enter the study is scheduled to complete screening in May 2025. First results from the study are expected in the second half of 2026.
CUV105: first SCENESSE® Phase III vitiligo trial
SCENESSE® is being evaluated as a systemic repigmentation therapy for vitiligo patients, with a clinical focus on adolescent (12 years and above) and adult patients with darker skin types (Fitzpatrick III-VI).1
CUV105 is a randomised multi-centre Phase III trial being conducted in 37 study sites across three continents. The majority of patients (
The vitiligo area scoring index (VASI) is being used to evaluate the primary endpoint, whereby the objective is to achieve a minimum of
Secondary endpoints include evaluations of repigmentation of face, neck and head (F-VASI) at week 20 and the maintenance of repigmentation following treatment completion. Patient Reported Outcomes deploy validated tools to assess patient perception of change (Patient Global Impression of Change in the face and the body and the Vitiligo Noticeability Scale) and quality of life (VitiQoL).
More than 200 patients have enrolled in CUV105 and commenced the treatment protocol.
Patients assigned to NB-UVB monotherapy are additionally eligible to receive SCENESSE® and adjuvant NB-UVB after completion of the follow-up period. CLINUVEL altered the CUV105 protocol at the end of last year to incorporate this extension period after requests from study sites and patients.
CLINICAL OBSERVATIONS
Clinical observations from CUV105 have been presented to global medical congresses, including the 2024 and 2025 American Academy of Dermatology Meetings, with further abstracts submitted for conferences later in 2025.
Four previously released case studies – patients with skin type IV and varying disease duration – demonstrated repigmentation of vitiliginous lesions on the face or back after four weeks of commencing treatment with afamelanotide, and that some patients experience additional spontaneous repigmentation following the conclusion of the treatment protocol. A fifth case study shared today, demonstrates repigmentation of lesions on the arms and legs in a skin type V patient with a 20-year history of the vitiligo.
All case study patients have reported satisfaction with the treatment results and that afamelanotide was well tolerated with adjunct NB-UVB.
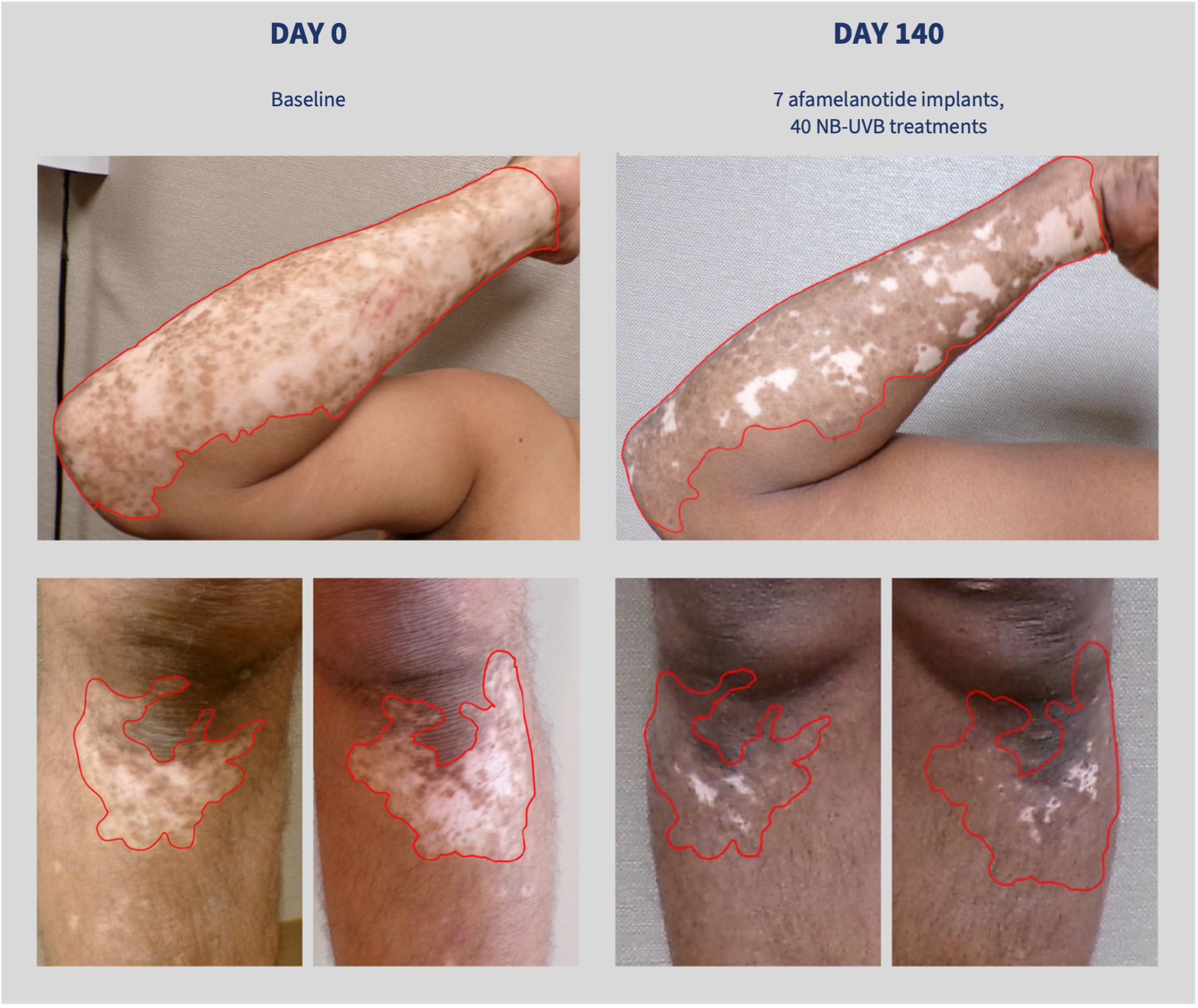
Above: the fifth case study to be released from CUV105, a 46-year-old male with skin type V treated with seven SCENESSE® implants and 40 NB-UVB sessions. The images demonstrate repigmentation of vitiliginous lesions on the patient’s right forearm (top) and lower legs (bottom). The patient was first diagnosed with vitiligo in 2004. The red outlines demonstrate the extent of the initially affected skin; the images are otherwise unaltered.
COMMENTARY
“We are thrilled with this enrolment milestone since we are essentially establishing a North American distribution network among dermatologists, anticipating the necessary infrastructure ahead of market entry of our breakthrough product,” CLINUVEL’s Director, Global Clinical Affairs, Dr Emilie Rodenburger said.
“The first clinical observations of the systemic (total body) solution are encouraging, and now we will continue a regulatory discussion in Europe, Africa and North America, all in anticipation of CUV107, a second large trial evaluating the treatment effects of SCENESSE® in vitiligo.”
ABOUT VITILIGO
Vitiligo is an acquired depigmentation disorder affecting 1
While the disease can have an impact on all patients, it is recognised that those with darker skin types are most severely affected. In North America, an estimated 820,000 individuals of darker skin types (IV-VI) are affected by vitiligo. The precise cause of the sudden start of this disorder remains unknown.
There is only one pharmaceutical product, a topical immunosuppressant, approved for vitiligo in the United States and Europe. Vitiligo patients with less than
Notes
The first four case reports from the CUV105 clinical trial were released to the ASX on 20 January 2025 and can be accessed here. CLINUVEL thanks the patients and treating physicians for being able to share these case reports.
1 The Fitzpatrick Skin Type is a numerical classification of human skin colour, from type I skin that always burns, to type VI, dark skin that never burns.
SCENESSE® for vitiligo patients: peer-reviewed research and conference presentations
Elbuluk, N. (2024). What’s New in Vitiligo. 2024 Meeting of the American Academy of Dermatology. 11 March. San Diego, USA.
Grimes, P. E., et al, (2013). The Efficacy of Afamelanotide and Narrowband UV-B Phototherapy for Repigmentation of Vitiligo. JAMA Dermatol, 149(1), 68–73.
Grimes, P.E. (2024). What’s new and hot in pigmentary disorders. 2024 Meeting of the American Academy of Dermatology. 9 March. San Diego, USA.
Kamangar, F. (2025) Afamelanotide: A Novel Promising Treatment for Vitiligo – Case Studies from the Randomized CUV105 Clinical Trial. Global Vitiligo Foundation Annual Scientific Symposium. 6 March. Orlando, USA.
Lim, H. W., et al., (2015). Afamelanotide and Narrowband UV-B Phototherapy for the Treatment of Vitiligo: A Randomized Multicenter Trial. JAMA Dermatol, 151(1), 42.
Lim, H.W. (2024). Clarence S. Livingood, MD Memorial Award and Lectureship: Photodermatology: Past, Present and Future. 2024 Meeting of the American Academy of Dermatology. 10 March. San Diego, USA.
Toh, J. J. H., et al, (2020). Afamelanotide implants and narrow-band ultraviolet B phototherapy for the treatment of nonsegmental vitiligo in Asians. J Am Acad Dermatol, 82(6), 1517–1519.
About CLINUVEL PHARMACEUTICALS LIMITED
CLINUVEL (ASX: CUV; ADR LEVEL 1: CLVLY; Börse Frankfurt: UR9) is a global specialty pharmaceutical group focused on developing and commercialising treatments for patients with genetic, metabolic, systemic, and life-threatening, acute disorders, as well as healthcare solutions for specialised populations. As pioneers in photomedicine and the family of melanocortin peptides, CLINUVEL’s research and development has led to innovative treatments for patient populations with a clinical need for systemic photoprotection, assisted DNA repair, repigmentation and acute or life-threatening conditions who lack alternatives.
CLINUVEL’s lead therapy, SCENESSE® (afamelanotide 16mg), is approved for commercial distribution in Europe, the USA, Israel, and Australia as the world’s first systemic photoprotective drug for the prevention of phototoxicity (anaphylactoid reactions and burns) in adult patients with erythropoietic protoporphyria (EPP). Headquartered in Melbourne, Australia, CLINUVEL has operations in Europe, Singapore, and the USA. For more information, please go to https://www.clinuvel.com.
Authorised for ASX release by the Board of Directors of CLINUVEL PHARMACEUTICALS LTD.
Head of Investor Relations
Mr Malcolm Bull, CLINUVEL PHARMACEUTICALS LTD
Investor Enquiries
https://www.clinuvel.com/investors/contact-us
Forward-Looking Statements
This release contains forward-looking statements, which reflect the current beliefs and expectations of CLINUVEL’s management. Statements may involve a number of known and unknown risks that could cause our future results, performance, or achievements to differ significantly from those expressed or implied by such forward-looking statements. Important factors that could cause or contribute to such differences include risks relating to: our ability to develop and commercialise pharmaceutical products; the COVID-19 pandemic and/or other world, regional or national events affecting the supply chain for a protracted period of time, including our ability to develop, manufacture, market and sell biopharmaceutical and PhotoCosmetic products; competition for our products, especially SCENESSE® (afamelanotide 16mg), CYACÊLLE, PRÉNUMBRA®, NEURACTHEL® or products developed and characterised by us as PhotoCosmetics; our ability to achieve expected safety and efficacy results in a timely manner through our innovative R&D efforts; the effectiveness of our patents and other protections for innovative products, particularly in view of national and regional variations in patent laws; our potential exposure to product liability claims to the extent not covered by insurance; increased government scrutiny in either Australia, the U.S., Europe, the UK, Israel, China, Japan, and/or LATAM regions of our agreements with third parties and suppliers; our exposure to currency fluctuations and restrictions as well as credit risks; the effects of reforms in healthcare regulation and pharmaceutical pricing and reimbursement; that the Company may incur unexpected delays in the outsourced manufacturing of SCENESSE®, CYACÊLLE, PRÉNUMBRA®, NEURACTHEL® or products developed as PhotoCosmetics which may lead to the Company being unable to launch, supply or serve its commercial markets, special access programs and/or clinical trial programs; any failures to comply with any government payment system (i.e. Medicare, Medicaid, and U.S. Department of Veteran’s Affairs) reporting and payment obligations; uncertainties surrounding the legislative and regulatory pathways for the registration and approval of biotechnology, cosmetic and consumer based products; decisions by regulatory authorities regarding approval of our products as well as their decisions regarding label claims; our ability to retain or attract key personnel and managerial talent; the impact of broader change within the pharmaceutical industry, cosmetic industry and related industries; potential changes to tax liabilities or legislation; environmental risks; and other factors that have been discussed in our 2024 Annual Report. Forward-looking statements speak only as of the date on which they are made, and the Company undertakes no obligation, outside of those required under applicable laws or relevant listing rules of the Australian Securities Exchange, to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. More information on preliminary and uncertain forecasts and estimates is available on request, whereby it is stated that past performance is not an indicator of future performance.
Contact:
Tel: +61 3 9660 4900
Fax: +61 3 9660 4909
Email: mail@clinuvel.com
Australia (Head Office), Level 22, 535 Bourke Street, Melbourne, Victoria, 3000, Australia
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/db406f89-4bf4-4767-849c-269ffea46d9a








