CRISPR Therapeutics and Sirius Therapeutics Announce Multi-Target Collaboration to Develop Novel siRNA Therapies
Rhea-AI Summary
Positive
- Strong Phase 1 clinical results showing >93% reduction in FXI levels and maintained efficacy for up to 6 months
- Strategic expansion of CRISPR's therapeutic portfolio beyond gene-editing into siRNA therapies
- 50-50 profit sharing agreement for SRSD107 with clear market division (US vs Greater China)
- Rights to develop two additional siRNA programs, expanding potential pipeline
- Large addressable market including multiple conditions (atrial fibrillation, VTE, cancer-associated thrombosis)
Negative
- Significant upfront payment of $95 million required from CRISPR Therapeutics
- Early-stage development with Phase 2 trials just beginning
- Competitive market with existing Factor Xa inhibitors and other anti-Factor XI modalities
News Market Reaction
On the day this news was published, CRSP gained 1.47%, reflecting a mild positive market reaction.
Data tracked by StockTitan Argus on the day of publication.
-Collaboration brings together complementary capabilities to co-develop and co-commercialize SRSD107, a next generation, long-acting Factor XI (FXI) small interfering RNA (siRNA) for the treatment of thromboembolic disorders-
-SRSD107 demonstrated peak reductions in FXI activity >
-Under the agreement, CRISPR Therapeutics will make an upfront payment of
-Expands CRISPR’s therapeutic toolkit to develop a broader range of transformative gene-based medicines in addition to the gene-editing programs in the clinic-
ZUG, Switzerland and BOSTON and SAN DIEGO and SHANGHAI, May 19, 2025 (GLOBE NEWSWIRE) -- CRISPR Therapeutics (NASDAQ: CRSP), a biopharmaceutical company focused on creating transformative gene-based medicines for serious diseases, and Sirius Therapeutics, a clinical stage biotech company developing innovative small interfering RNA (siRNA) therapies for global markets, today announced a strategic partnership to develop and commercialize siRNA therapies.
“We are excited to partner with Sirius, and broaden our cardiovascular medicine portfolio, on the heels of promising top-line data that we recently shared for CTX310, which targets ANGPTL3,” said Samarth Kulkarni, Ph.D., Chairman and Chief Executive Officer of CRISPR Therapeutics. “Coagulation Factor XI represents an innovative and highly compelling target for treating thrombotic diseases that affect millions worldwide. SRSD107, which targets Factor XI, has the potential to be a best-in-class therapy, offering infrequent dosing and improved patient outcomes. Sirius’ siRNA platform complements our existing capabilities and expands our therapeutic toolkit, enabling us to develop a broader range of transformative gene-based medicines.”
“We are pleased to collaborate with CRISPR Therapeutics, a recognized leader in the development of gene-based medicines,” said Qunsheng Ji, MD, Ph.D. Chief Executive Officer of Sirius Therapeutics. “Thrombotic diseases represent a significant unmet need, and our promising Phase 1 data highlights the potential of SRSD107 as a best-in-class Factor XI-targeted therapy. Sirius is committed to addressing the needs of these patients, as we work with CRISPR Therapeutics to advance novel siRNA therapies globally.”
“There is a large population of patients who are at risk for potentially life-threatening thromboembolic events due to underlying co-morbid diseases such as malignancy, cardiovascular disease, and hyper-coagulability. A significant percentage of these patients are inadequately treated due to concerns for bleeding risk, or challenges with compliance,” said Christian T. Ruff, M.D., M.P.H., senior investigator of TIMI Group, director General Cardiology, Brigham and Women’s Hospital, and associate professor, Harvard Medical School. “SRSD107 offers the potential for a therapy with lower bleeding risk, infrequent dosing for better compliance, without concerns for renal clearance or drug interactions, and reversibility to further mitigate bleeding risks that could be differentiated from currently available therapies and other Factor XI modalities.”
SRSD107 is a next generation, long-acting siRNA designed to selectively inhibit Factor XI (FXI), a key driver of pathological thrombosis with minimal impact on normal hemostasis. By targeting FXI, SRSD107 aims to reduce thrombotic events while minimizing the risk of bleeding – representing a differentiated approach compared to Factor Xa inhibitors. In addition, SRSD107 may offer the potential for reversibility not observed with other anti-Factor XI modalities. The addressable population includes patients with atrial fibrillation, venous thromboembolism (VTE), cancer-associated thrombosis, chronic Coronary Artery Disease (CAD), chronic Peripheral Vascular Disease (PVD), end-stage renal disease requiring hemodialysis, and patients undergoing major orthopedic surgery, where bleeding risk limits existing therapies.
The clinical program for SRSD107 includes two promising Phase 1 clinical trials, where single doses of SRSD107 were found to be safe and well tolerated. In addition, SRSD107 demonstrated robust pharmacodynamic effects, including reductions of over
Figure 1. SRSD107 Phase 1 Clinical Results: Sustained, dose-dependent pharmacodynamic response to therapy
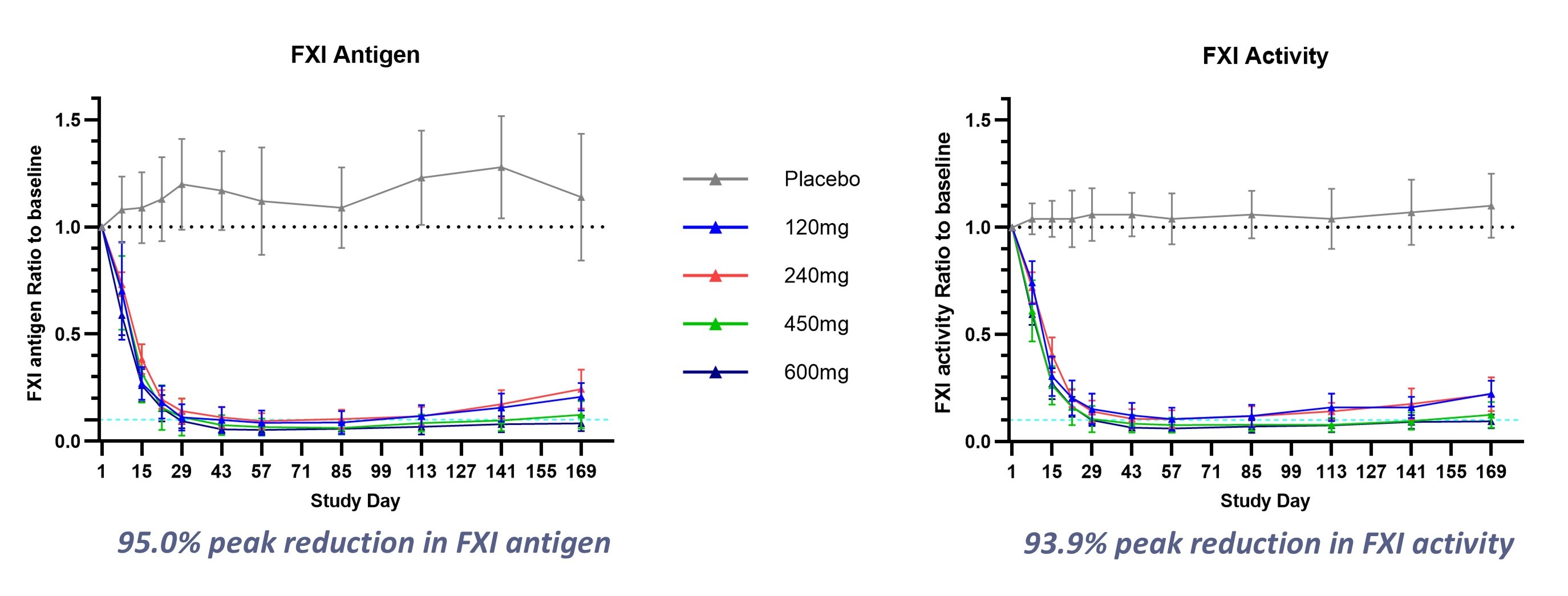
A Phase 2 clinical trial of SRSD107 is being initiated to evaluate its safety and efficacy for the prevention of VTE in patients undergoing total knee arthroplasty. The trial aims to confirm the anticoagulant benefits of SRSD107 and to inform dose selection for future pivotal trials.
Collaboration Details
Under the terms of the agreement, CRISPR Therapeutics will make an upfront payment of
Additionally, CRISPR Therapeutics will have the option to nominate up to two siRNA targets for research and development. For each target, CRISPR Therapeutics will fund research and retain opt-in rights to lead clinical development and commercialization. Sirius will be eligible to receive milestone payments, as well as tiered royalties ranging from high single to low-double digits.
About CRISPR Therapeutics
Since its inception over a decade ago, CRISPR Therapeutics has evolved from a research-stage company advancing gene editing programs into a leader that celebrated the historic approval of the first-ever CRISPR-based therapy. The Company has a diverse portfolio of product candidates across a broad range of disease areas including hemoglobinopathies, oncology, regenerative medicine, cardiovascular, autoimmune, and rare diseases. In 2018, CRISPR Therapeutics advanced the first-ever CRISPR/Cas9 gene-edited therapy into the clinic to investigate the treatment of sickle cell disease and transfusion-dependent beta thalassemia. Beginning in late 2023, CASGEVY® (exagamglogene autotemcel [exa-cel]) was approved in several countries to treat eligible patients with either of these conditions. The Nobel Prize-winning CRISPR technology has revolutionized biomedical research and represents a powerful, clinically validated approach with the potential to create a new class of potentially transformative medicines. To accelerate and expand its efforts, CRISPR Therapeutics has formed strategic partnerships with leading companies including Vertex Pharmaceuticals. CRISPR Therapeutics AG is headquartered in Zug, Switzerland, with its wholly-owned U.S. subsidiary, CRISPR Therapeutics, Inc., and R&D operations based in Boston, Massachusetts and San Francisco, California. To learn more, visit www.crisprtx.com.
CRISPR THERAPEUTICS® standard character mark and design logo, CTX310™ and CTX320™ are trademarks and registered trademarks of CRISPR Therapeutics AG. CASGEVY® and the CASGEVY logo are registered trademarks of Vertex Pharmaceuticals Incorporated. All other trademarks and registered trademarks are the property of their respective owners.
CRISPR Therapeutics Forward-Looking Statement
Statements contained in this press release regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Such statements include, but are not limited to, statements made by Drs. Kulkarni, Ji and Ruff in this press release, as well as regarding any or all of the following: (i) CRISPR Therapeutics preclinical studies, clinical trials and pipeline products and programs, including, without limitation, expectations regarding data, safety and efficacy generally; (ii) data included in this press release, as well as the ability to use data from ongoing and planned clinical trials for the design and initiation of further clinical trials; (iii) the status and clinical progress of the SRSD107 clinical program and development timelines for such program; (iv) CRISPR Therapeutics strategy and goals; (v) the future activities of the parties pursuant to the collaboration and the expected benefits of CRISPR Therapeutics’ collaboration with Sirius Therapeutics; and (vi) the therapeutic value, development, and commercial potential of gene editing and delivery technologies and therapies, including CRISPR/Cas9. Risks that contribute to the uncertain nature of the forward-looking statements include, without limitation, the risks and uncertainties discussed under the heading “Risk Factors” in its most recent annual report on Form 10-K and in any other subsequent filings made by CRISPR Therapeutics with the U.S. Securities and Exchange Commission. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date they are made. We disclaim any obligation or undertaking to update or revise any forward-looking statements contained in this press release, other than to the extent required by law.
This press release discusses investigational therapies and is not intended to convey conclusions about efficacy or safety as to those investigational therapies or uses of such investigational therapies. There is no guarantee that any investigational therapy will successfully complete clinical development or gain approval from applicable regulatory authorities.
About Thromboembolic Disorders
Thrombosis, or blood clot formation, is the common underlying mechanism of most cases of myocardial infarction, ischemic stroke, and venous thromboembolism. According to a trial in The Lancet1 of regional and global mortality rates, thromboembolic disorders are estimated to cause as many as 1 in 4 deaths worldwide.
About SRSD107
SRSD107 is a novel double-stranded small interfering ribonucleic acid (siRNA). SRSD107 specifically targets the human coagulation factor XI (FXI) mRNA and inhibits FXI protein expression, thereby blocking the intrinsic coagulation pathway and promoting anticoagulant/anti-thrombotic effects. SRSD107 has been engineered for the potential to enable twice-a-year dosing.
About Sirius Therapeutics
Sirius is a clinical stage biotech company developing innovative siRNA therapies for global markets. We are dedicated to discovering and developing new treatment options for cardiovascular and cerebrovascular disease and translating siRNA technology into transformative medicine for chronic disease patients. Sirius’s most advanced products are SRSD107 for the treatment of thromboembolic disorders, SRSD216 for the treatment of hyperlipoproteinemia, and SRSD101 for the treatment of dyslipidemia.
Founded in 2021 by a world-class leadership team and investors, Sirius has established an innovation center in the United States and translational medicine center in China. Sirius has raised nearly US
References:
1. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380, 2095-1128.
Investor Contact:
+1-617-307-7503
ir@crisprtx.com
Media Contact:
+1-617-315-4493
media@crisprtx.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/ef059750-fffd-455a-bca7-bd40c7b03378








