First Set of 1-Year Clinical Results from RESOLVE Trial in Eosinophilic Esophagitis (EoE), Durable and Sustained Symptom & Tissue Responses after Dosing with EP-104GI
Rhea-AI Summary
Eupraxia Pharmaceuticals (NASDAQ:EPRX) has announced positive 1-year clinical results from its Phase 1b/2a RESOLVE trial evaluating EP-104GI for treating eosinophilic esophagitis (EoE). The trial demonstrated that 67% of patients in Cohort 5 (48mg dose) maintained clinical remission after 12 months.
Key findings include significant tissue health improvements, with patients treated with 4mg per injection showing 47% improvement in grade and 44% in stage at week 36. The drug maintained steady plasma levels through 52 weeks, below typical asthma inhaler levels. Importantly, no serious adverse events or oral/gastrointestinal candidiasis were reported.
The company believes EP-104GI could potentially offer a revolutionary once-yearly treatment option, administered during routine annual endoscopy procedures, presenting a significant advantage over current daily oral steroids or weekly biologics.
Positive
- None.
Negative
- Limited patient data for 52-week results (n=3 for clinical remission data)
- Trial still ongoing with full results pending
News Market Reaction
On the day this news was published, EPRX gained 0.93%, reflecting a mild positive market reaction. Argus tracked a trough of -5.5% from its starting point during tracking. This price movement added approximately $2M to the company's valuation, bringing the market cap to $195M at that time. Trading volume was very high at 3.2x the daily average, suggesting strong buying interest.
Data tracked by StockTitan Argus on the day of publication.
- At 12 months, 2/3rds of Cohort 5 patients (48mg dose, 4mg per site) remained in clinical remission after their treatment with EP-104GI
- All Cohorts followed to 9 months have maintained clinically meaningful improvements in tissue health as measured by EoE Histological Scoring System (“EoEHSS”)
- No Serious Adverse Events (“SAE”) or any events of oral or gastrointestinal candidiasis have been reported to date in the entire trial
VICTORIA, British Columbia, Sept. 02, 2025 (GLOBE NEWSWIRE) -- Eupraxia Pharmaceuticals Inc. (“Eupraxia” or the “Company”) (NASDAQ:EPRX) (TSX:EPRX), a clinical-stage biotechnology company leveraging its proprietary Diffusphere™ technology designed to optimize local, controlled drug delivery for applications with significant unmet need, today announced additional positive clinical data from its ongoing Phase 1b/2a RESOLVE trial evaluating EP-104GI for the treatment of eosinophilic esophagitis (“EoE”), including the first clinical data measured 52 weeks after patients were treated with EP-104GI.
“We believe the prolonged duration of symptom response that we are seeing with EP-104GI is truly a unique clinical result and will potentially provide a once-a-year therapy to patients with EoE. And overall, we continue to see that the more drug we deliver to the tissues, the better the results,” said Dr. James A. Helliwell, Chief Executive Officer of Eupraxia. “Independent market research has shown that the majority of patients with EoE undergo a routine endoscopy at least once a year to monitor the progress of their disease, which is also consistent with national guidelines. Based on this, leading KOLs in EoE see a potential treatment regimen where EP-104GI is administered during this routine annual procedure, in contrast to current standards of care which are inconvenient and involve swallowing oral steroids daily or injecting themselves weekly with a biologic. As a result, we believe EP-104GI has the potential to significantly enhance the current standard of care for patients with EoE. We look forward to reporting additional 12-month data from a larger patient set later this year.”
Key Findings from the 4mg Dose Groups in the Phase 1b/2a RESOLVE trial
- Symptoms
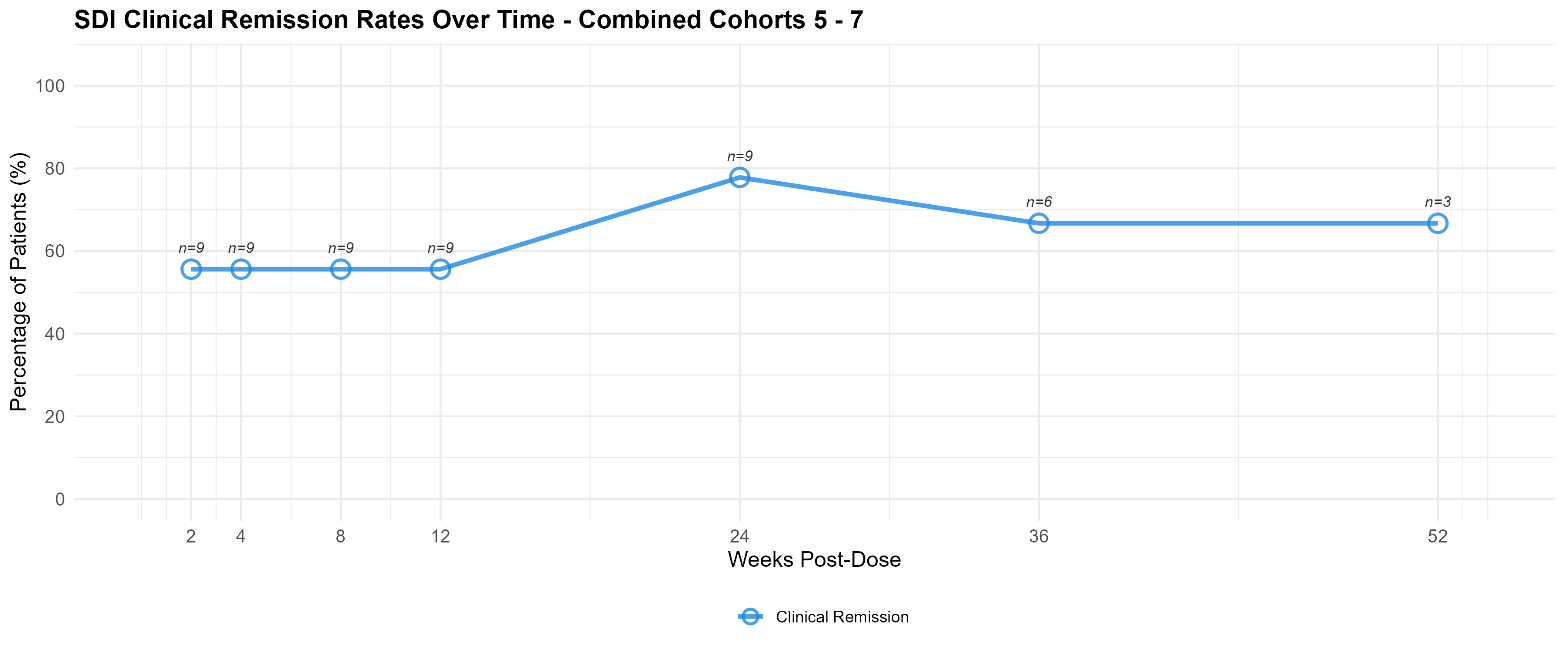
- Patients treated with 4mg per injection of EP-104GI had meaningful levels of clinical remission as measured by Straumann Dysphagia Index (“SDI”) at Week 12 (
56% , n=9), Week 24 (78% , n=9), Week 36 (67% , n=6) and Week 52 (67% , n=3). See graph below.
- Patients treated with 4mg per injection of EP-104GI had meaningful levels of clinical remission as measured by Straumann Dysphagia Index (“SDI”) at Week 12 (
SDI Clinical Remission Rates Over Time, 4mg per Injection Group (Cohorts 5-7)
- Tissue Health
- Patients treated with 4mg per injection (n=6) of EP-104GI demonstrated mean improvements in EoEHSS of
47% in grade (severity) and44% in stage (extent) at week 36, continuing the trend of durable improvements in tissue health. Across dose groups, there is a significant correlation between pharmacokinetics and changes in tissue health.
- Patients treated with 4mg per injection (n=6) of EP-104GI demonstrated mean improvements in EoEHSS of
- Pharmacokinetics
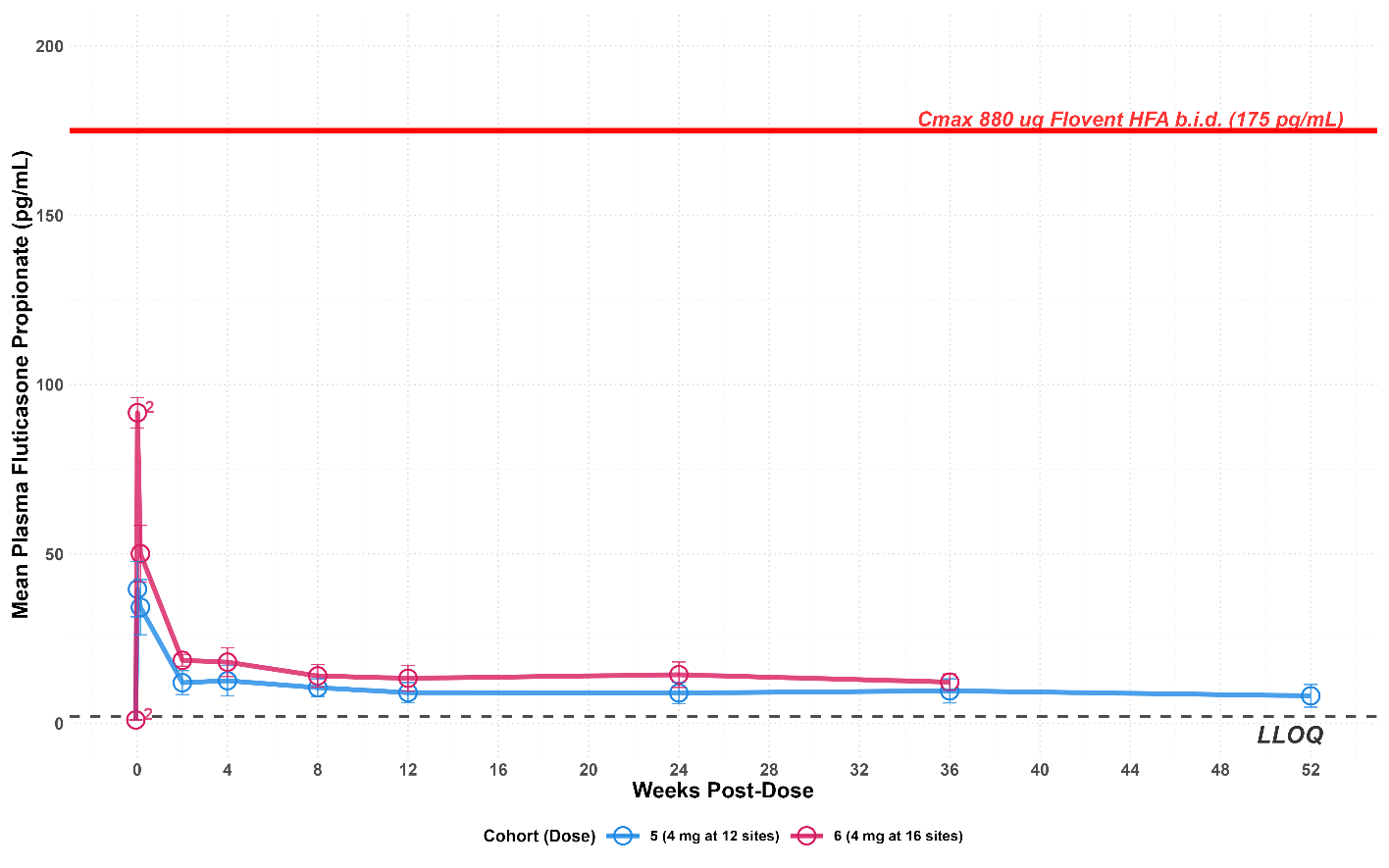
- Plasma levels of fluticasone in patients treated with 4mg per injection of EP-104GI remained level and predictable out to 52 weeks (see graph below). This is well below the levels typically observed with daily asthma inhalers which is shown as the red line on the graph. There have been no SAEs or incidence of either oral or gastrointestinal candidiasis reported to date.
EP-104GI Pharmacokinetics – Week 52 levels remain steady
An updated summary of the above and previously announced clinical trial results are posted in the Investor Section of the Eupraxia Pharmaceuticals website and can be found here.
Notes
- Straumann Dysphagia Index (“SDI”) is a patient-reported outcome score that uses a seven-day recall measuring dysphagia (trouble swallowing) severity and frequency. A reduction in SDI is a positive outcome for the RESOLVE trial.
- In the EoE Histological Scoring System (“EoEHSS”), grade indicates the severity of each of the eight histologic features assessed by the EoEHSS, while stage indicates their extent. The EoEHSS has two broad categories of tissue examination; Inflammatory components (driven by Eosinophils) and Architectural components (Fibrosis and tissue health)., A reduction in EoEHSS is a positive outcome for the RESOLVE trial.
- Peak Eosinophil Count (“PEC”) is a measure of the peak number of eosinophils found in esophageal biopsies. Eosinophils are one of several white blood cells that support a person’s immune system. A reduction in PEC is a positive outcome for the RESOLVE trial. If a biopsy site has less than or equal to 6 eosinophils, that site is considered to be in remission. Remission Rate is the percentage of biopsies that are in remission.
About the RESOLVE Trial
The RESOLVE trial is a Phase 1b/2a, multicenter, open-label, dose-escalation study evaluating the safety, tolerability, pharmacokinetics, and efficacy of EP-104GI in adults with histologically confirmed active EoE. The treatment is administered as a single dose via 4 to 20 esophageal wall injections, with dose escalations modifying either the dose per site and/or the number of sites. Participants were followed for up to 24 weeks (4x1mg, 8x1mg, 8x2.5mg and 12x2.5mg) or 52 weeks (12x4mg and subsequent ongoing dose levels). Eupraxia plans to disclose additional data from the open label Phase 1b/2a part of the RESOLVE trial in Q3 2025.
The Phase 2b part of the RESOLVE trial, a randomized placebo-controlled study of EP-104GI, is currently recruiting with the first clinical dose of 120mg (20 x 6mg). The top-line data from the Phase 2b part of the RESOLVE trial is expected in Q3 2026.
About Eosinophilic Esophagitis (EoE)
EoE is an inflammatory-mediated disease in which white blood cells migrate into and become trapped in the esophagus, creating pain and difficulty with swallowing food. According to market research from Clearview Healthcare Partners, EoE affects more than 450,000 people in the United States and has been identified by the American Gastroenterological Association as rapidly increasing in both incidence and prevalence. Impacts from both symptoms and interventions frequently lead to mental health issues, compounding the disease burden of EoE for both the healthcare system and the individual.
About Eupraxia Pharmaceuticals Inc.
Eupraxia is a clinical-stage biotechnology company focused on the development of locally delivered, extended-release products that have the potential to address therapeutic areas with high unmet medical need. Diffusphere™, a proprietary, polymer-based micro-sphere technology, is designed to facilitate targeted drug delivery of both existing and novel drugs. The technology is designed to support extended duration of effect and delivery of drugs in a hyper-localized fashion, targeting only the tissues that physicians are wanting to treat. We believe the potential for fewer adverse events may be achieved through the precision targeting and the stable and flat delivery of the active ingredient when using the Diffusphere™ technology, versus the peaks and troughs seen with more traditional drug delivery methods. The precision of Eupraxia's Diffusphere™ technology platform has the potential to augment and transform existing FDA-approved drugs to improve their safety, tolerability, efficacy and duration of effect. The potential uses in therapeutic areas may go beyond pain and inflammatory gastrointestinal disease, where Eupraxia currently is developing advanced treatments, to also be applicable in oncology, infectious disease and other critical disease areas.
Eupraxia's EP-104GI is currently in a Phase 1b/2 trial, the RESOLVE trial, for the treatment of EoE. EP-104GI is administered as an injection into the esophageal wall, providing local delivery of drug. This is a unique treatment approach for EoE. Eupraxia also recently completed a Phase 2b clinical trial (SPRINGBOARD) of EP-104IAR for the treatment of pain due to knee osteoarthritis. The trial met its primary endpoint and three of the four secondary endpoints. In addition, Eupraxia is developing a pipeline of later and earlier-stage long-acting formulations. Potential pipeline indications include candidates for other inflammatory joint indications and oncology, each designed to improve on the activity and tolerability of currently approved drugs. For further details about Eupraxia, please visit the Company's website at: www.eupraxiapharma.com.
Notice Regarding Forward-looking Statements and Information
This news release includes forward-looking statements and forward-looking information within the meaning of applicable securities laws. Often, but not always, forward-looking information can be identified by the use of words such as "plans", "is expected", "expects", "suggests", "scheduled", "intends", "contemplates", "anticipates", "believes", "proposes", "potential" or variations (including negative and grammatical variations) of such words and phrases, or statements that certain actions, events or results "may", "could", "would", "might" or "will" be taken, occur or be achieved. Forward-looking statements in this news release include statements regarding the Company’s expected timing of reporting additional data from the RESOLVE trial in Q3 2025; the Company's product candidates, including their expected benefits to patients with respect to safety, tolerability, efficacy and duration; the expectations around proceeding to clinical trials for the Company’s product candidates; the results gathered from studies and trials of Eupraxia's product candidates; the potential for the Company’s technology to impact the drug delivery process; potential market opportunity for the Company’s product candidates; and potential pipeline indications. Such statements and information are based on the current expectations of Eupraxia's management, and are based on assumptions, including but not limited to: future research and development plans for the Company proceeding substantially as currently envisioned; industry growth trends, including with respect to projected and actual industry sales; the Company's ability to obtain positive results from the Company's research and development activities, including clinical trials; and the Company's ability to protect patents and proprietary rights. Although Eupraxia's management believes that the assumptions underlying these statements and information are reasonable, they may prove to be incorrect. The forward-looking events and circumstances discussed in this news release may not occur by certain dates or at all and could differ materially as a result of known and unknown risk factors and uncertainties affecting Eupraxia, including, but not limited to: risks and uncertainties related to the Company's limited operating history; the Company's novel technology with uncertain market acceptance; if the Company breaches any of the agreements under which it licenses rights to its product candidates or technology from third parties, the Company could lose license rights that are important to its business; the Company's current license agreement may not provide an adequate remedy for its breach by the licensor; the Company's technology may not be successful for its intended use; the Company's future technology will require regulatory approval, which is costly and the Company may not be able to obtain it; the Company may fail to obtain regulatory approvals or only obtain approvals for limited uses or indications; the Company's clinical trials may fail to demonstrate adequately the safety and efficacy of its product candidates at any stage of clinical development; the Company may be required to suspend or discontinue clinical trials due to side effects or other safety risks; the Company completely relies on third parties to provide supplies and inputs required for its product candidates and services; the potential impact of tariffs on the cost of the Company’s active pharmaceutical ingredients and clinical supplies of EP-104IAR and EP-104GI; the Company relies on external contract research organizations to provide clinical and non-clinical research services; the Company may not be able to successfully execute its business strategy; the Company will require additional financing, which may not be available; any therapeutics the Company develops will be subject to extensive, lengthy and uncertain regulatory requirements, which could adversely affect the Company's ability to obtain regulatory approval in a timely manner, or at all; the impact of health pandemics or epidemics on the Company's operations; the Company's restatement of its consolidated financial statements, which may lead to additional risks and uncertainties, including loss of investor confidence and negative impacts on the Company's common share price; and other risks and uncertainties described in more detail in Eupraxia's public filings on SEDAR+ (sedarplus.ca) and EDGAR (sec.gov). Although Eupraxia has attempted to identify important factors that could cause actual actions, events or results to differ materially from those described in forward-looking statements and information, there may be other factors that cause actions, events or results to differ from those anticipated, estimated or intended. No forward-looking statement or information can be guaranteed. Except as required by applicable securities laws, forward-looking statements and information speak only as of the date on which they are made and Eupraxia undertakes no obligation to publicly update or revise any forward-looking statement or information, whether as a result of new information, future events or otherwise.
For investor and media inquiries, please contact:
Danielle Egan, Eupraxia Pharmaceuticals Inc.
778.401.3302
degan@eupraxiapharma.com
or
Kevin Gardner, on behalf of:
Eupraxia Pharmaceuticals Inc.
617.283.2856
kgardner@lifesciadvisors.com
SOURCE Eupraxia Pharmaceuticals Inc.
Photos accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/bb4ddba4-14db-422a-ac36-31e42f6cb5d1
https://www.globenewswire.com/NewsRoom/AttachmentNg/a386eff3-131c-49d8-b173-77aea021c4a9








