Hyperfine Announces FDA Clearance of a New Next-Generation Swoop® System Powered by Optive AI™ Software, Delivering a Transformative Leap in Image Quality
This major Swoop® system scanner redesign and Optive AI™ software elevate the AI-powered portable MRI experience for clinicians and their patients across multiple sites of care.
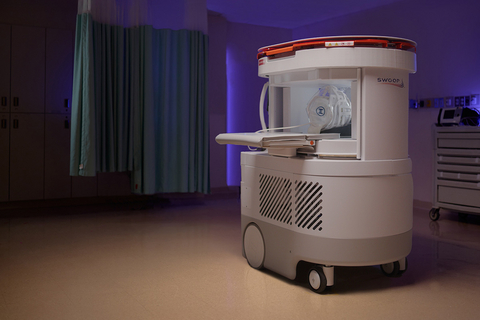
The New Next-Generation Swoop® AI-Powered Portable MRI System
The next-generation Swoop® system incorporates learnings from five years of real-world experience across seven continents and numerous care settings. The new Swoop® system features innovations specifically engineered to deliver the highest signal-to-noise ratio, which, when paired with the Optive AI™ software, achieve exceptional image quality, including improved resolution, uniformity, and faster acquisition times. This new level of image quality has the potential to dramatically drive the adoption of the Swoop® system across multiple sites of care and clinical applications. The new Swoop® system also delivers a user and patient-centric design to accommodate a broad patient population—especially beneficial for pediatric, elderly, or anxious patients—making MRI more accessible for all. With the optimized user experience, the Swoop® system now, more than ever, empowers clinicians to acquire scans when and where it matters most.
“This next-generation hardware and the Optive AI™ software platform begin a new chapter for the adoption of AI-powered portable MRI. This clearance is the most significant innovation in the history of Hyperfine. We plan to launch the new Swoop® system across hospital and office settings in the US, and we believe the new Swoop® system performance will delight patients and providers, reduce the learning curve for users, and drive meaningfully faster adoption,” said Maria Sainz, President and CEO of Hyperfine.
Collaboration with leading portable MRI programs, including Jefferson Abington, has accelerated the development of the new system. These strong clinical partnerships have helped shape hardware and software refinements critical to clinical utility. As Jennifer Villa Frabizzio, MD, Neuroradiologist at the Radiology Group of Abington, noted, “This sleek, innovative redesign of the Swoop® system is a transformational advance for the emerging field of portable MR imaging. It improves patient comfort by accommodating a broader range of body types and increases efficiency by allowing technologists to reach patients more quickly. But the real breakthrough lies in how the hardware and software work together to deliver image quality and speed, which brings portable MRI into the realm of mainstream clinical practice."
The new Swoop® system is being used in NEURO PMR, a Hyperfine-sponsored study examining the clinical utility of portable MRI in neurology offices. The two participating sites have reported very positive feedback from both users and patients so far. “Based on my extensive experience in the NEURO PMR study, I believe the new Swoop® system marks a remarkable advancement in image quality while maintaining an affordable and compact design,” said Dr. Laszlo Mechtler, Medical Director of Dent Neurologic Institute and principal investigator of the NEURO PMR study. “It is a compelling solution for neurology practices who want to offer on-site, cost-effective brain imaging to enhance patient care, convenience, and comfort.”
With this launch, Hyperfine will turn a long-held vision to transform access to MRI globally into a clinical reality. By combining powerful AI with a reimagined portable MRI platform, the company is redefining how—and where—brain imaging can be delivered. The new Swoop® system accelerates Hyperfine's strategy to broaden adoption across a wide range of care environments, including critical care, emergency, and clinic settings in hospitals, neurology offices, and remote and rural care environments. It marks a pivotal step toward making high-quality brain imaging more accessible, efficient, and impactful wherever patients are.
For more information about the Swoop® Portable MR Imaging® system, please visit HyperfineMRI.com.
About the Swoop® AI-Powered Portable MRI (V2) System
The Swoop® Portable MR Imaging® (V2) System is
About Hyperfine, Inc.
Hyperfine, Inc. (Nasdaq: HYPR) is the groundbreaking health technology company that has redefined brain imaging with the Swoop® system—the first FDA-cleared, portable, ultra-low-field, magnetic resonance brain imaging system capable of providing imaging at multiple points of professional care. The mission of Hyperfine, Inc. is to revolutionize patient care globally through transformational, accessible, clinically relevant diagnostic imaging. Founded by Dr. Jonathan Rothberg in a technology-based incubator called 4Catalyzer, Hyperfine, Inc. scientists, engineers, and physicists developed the Swoop® system out of a passion for redefining brain imaging methodology and how clinicians can apply accessible diagnostic imaging to patient care. For more information, visit HyperfineMRI.com.
The Hyperfine logo, Swoop, and Portable MR Imaging are registered trademarks of Hyperfine, Inc. The Swoop logo, Optive AI logo, and Optive AI are trademarks of Hyperfine, Inc.
Forward-Looking Statements
This press release includes “forward-looking statements” within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. Actual results of Hyperfine, Inc. (the “Company”) may differ from its expectations, estimates and projections and consequently, you should not rely on these forward-looking statements as predictions of future events. Words such as “expect,” “estimate,” “project,” “budget,” “forecast,” “anticipate,” “intend,” “plan,” “may,” “will,” “could,” “should,” “believes,” “predicts,” “potential,” “continue,” and similar expressions (or the negative versions of such words or expressions) are intended to identify such forward-looking statements. These forward-looking statements include, without limitation, the Company’s goals and commercial plans, the benefits of the Company’s products and services, and the Company’s future performance and its ability to implement its strategy. These forward-looking statements involve significant risks and uncertainties that could cause the actual results to differ materially from the expected results. Most of these factors are outside of the Company’s control and are difficult to predict. Factors that may cause such differences include, but are not limited to: the success, cost and timing of the Company’s product development and commercialization activities, including the degree that the Swoop® system is accepted and used by healthcare professionals; the impact of COVID-19 on the Company’s business; the inability to maintain the listing of the Company’s Class A common stock on the Nasdaq; the Company’s inability to grow and manage growth profitably and retain its key employees; changes in applicable laws or regulations; the inability of the Company to raise financing in the future; the inability of the Company to obtain and maintain regulatory clearance or approval for its products, and any related restrictions and limitations of any cleared or approved product; the inability of the Company to identify, in-license or acquire additional technology; the inability of the Company to maintain its existing or future license, manufacturing, supply and distribution agreements and to obtain adequate supply of its products; the inability of the Company to compete with other companies currently marketing or engaged in the development of products and services that the Company is currently marketing or developing; the size and growth potential of the markets for the Company’s products and services, and its ability to serve those markets, either alone or in partnership with others; the pricing of the Company’s products and services and reimbursement for medical procedures conducted using the Company’s products and services; the Company’s estimates regarding expenses, revenue, capital requirements and needs for additional financing; the Company’s financial performance; and other risks and uncertainties indicated from time to time in Company’s filings with the Securities and Exchange Commission, including those under “Risk Factors” therein. The Company cautions readers that the foregoing list of factors is not exclusive and that readers should not place undue reliance upon any forward-looking statements, which speak only as of the date made. The Company does not undertake or accept any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements to reflect any change in its expectations or any change in events, conditions or circumstances on which any such statement is based.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250602409384/en/
Media Contact
Devin Zell
Hyperfine
dzell@hyperfine.io
Investor Contact
Webb Campbell
Gilmartin Group LLC
webb@gilmartinir.com
Source: Hyperfine, Inc.







