ImmunityBio Announces Durable Complete Response of 15 Months with a Chemotherapy-Free CD19 CAR-NK Cell Therapy in Waldenstrom Lymphoma
Key Terms
car-nk medical
rituximab medical
allogeneic medical
chimeric antigen receptor medical
natural killer cell medical
lymphodepletion medical
antibody-dependent cellular cytotoxicity medical
monoclonal antibody medical
- Durable complete responses with CAR-NK + Rituximab in patients who failed current standards of care in Waldenstrom’s Non-Hodgkins lymphoma (NHL).
- Long-term evaluable patients demonstrate ongoing complete response of 7 and 15 months after receiving a total of eight doses four cycles of CD19 CAR-NK plus rituximab, with no further treatment thereafter
-
The first chemotherapy free, lymphodepletion-free CAR- NK cell therapy demonstrating
100% disease control in first four subjects, all administered as outpatient therapy - QUILT-106 off-the-shelf CD19 CAR-NK trial is ongoing and represents a next-generation NK cell therapy for Non-Hodgkins Lymphoma
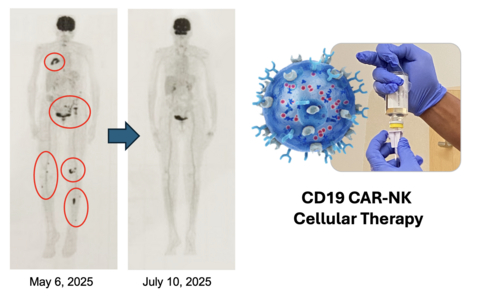
Accompanying figure is a PET scan of the complete response in the patient with multiple bone tumors and this CR is ongoing for seven months to date.
Updated follow-up demonstrates sustained complete responses with durations now extending to 15 months and ongoing, with
To date four patients with Waldenstrom Non-Hodgkin Lymphoma have been enrolled and all remain in clinical disease control. Two patients are evaluable for long-term follow-up and continue to demonstrate durable complete remission at 7 and 15 months and ongoing, respectively, despite receiving no additional treatment after the initial eight doses of immunotherapy.
Both the rapid onset of complete remission (after only two cycles) and the durability of complete response following treatment cessation underscores the potential for long-term immune-mediated disease control without continuous therapy.
“This updated follow-up reinforces the central thesis that restoring and activating the immune system can deliver durable control of disease without chemotherapy or lymphodepletion,” said Patrick Soon‑Shiong, MD, Founder, Executive Chairman, and Global Chief Medical and Scientific Officer of ImmunityBio. “Seeing complete responses persist beyond a year after treatment has stopped, in patients who had exhausted available options, represents a meaningful advance for patients with this rare disease of Waldenström lymphoma and validates CAR-NK as a potential next-generation immunotherapy platform.”
In two patients evaluable for long-term follow up who presented with extensive disease at baseline, one with multiple lymphomatous bone lesions and one with approximately
The patient with significant bone marrow involvement had complete bone morphological remission. In this patient in which
These findings represent the first chemotherapy-free and lymphodepletion-free immunotherapy regimen combining off-the-shelf allogeneic CD19 CAR-NK cells with rituximab to demonstrate
“These data highlight a favorable safety and efficacy profile that is particularly important for patients with indolent yet incurable lymphomas,” said Lennie Sender, M.D., Chief Medical Officer, Liquid Tumors and Cell Therapy at ImmunityBio. “To date, all patients have been treated as outpatients with no serious adverse events, demonstrating the feasibility of delivering potent cellular immunotherapy without the morbidity traditionally associated with cell-based treatments.”
Waldenström Non-Hodgkins lymphoma remains an area of significant unmet medical need, particularly for patients who relapse or become refractory to available targeted and antibody-based therapies. The updated results from QUILT-106 support off-the-shelf CD19 CAR-NK as a next-generation cell therapy for liquid tumors, combining durable efficacy with an outpatient-based treatment.
Enrollment and follow-up in QUILT-106 are ongoing, and additional clinical updates will be provided as more patients become evaluable and response durability continues to mature.
QUILT-106 was designed to examine CD19 CAR NK as a single experiential therapy combined with rituximab. A followup study is being designed to test the further combination of the NK-CAR with ANKTIVA (nogapendekin alfa inbakicept; N-803), a superagonist IL-15, and rituximab to build on the success of the QUILT-106 in indolent lymphoma including Waldenström’s Macroglobulinemia.
ImmunityBio’s CD19 CAR-NK Therapy
CD19 CAR-NK is a targeted high-affinity natural killer cell therapy – an off-the-shelf, allogeneic NK cell line engineered to express a CD19-specific chimeric antigen receptor (CAR) and a high-affinity CD16 (FcγRIIIa 158V) receptor. This design enables dual anti-tumor mechanisms: direct CAR-mediated cytotoxicity and augmented antibody-dependent cellular cytotoxicity when paired with anti-CD20 monoclonal antibody rituximab. Combining CD19 CAR-NK cells with rituximab could thereby target CD19⁺/CD20⁺ lymphoma cells to enhance tumor cell killing.
About ANKTIVA® (nogapendekin alfa inbakicept)
The cytokine interleukin-15 (IL-15) plays a crucial role in the immune system by affecting the development, maintenance, and function of key immune cells—NK and CD8+ killer T cells—that are involved in killing cancer cells. By activating NK cells, ANKTIVA® overcomes the tumor escape phase of clones resistant to T cells and restores memory T cell activity with resultant prolonged duration of complete response. A key component in the Company’s BioShield platform, ANKTIVA is a first-in-class IL-15 agonist IgG1 fusion complex, consisting of an IL-15 mutant (IL-15N72D) fused with an IL-15 receptor alpha, which binds with high affinity to IL-15 receptors on NK, CD4+, and CD8+ T cells. This fusion complex of ANKTIVA® mimics the natural biological properties of the membrane-bound IL-15 receptor alpha, delivering IL-15 by dendritic cells and driving the activation and proliferation of NK cells with the generation of memory killer T cells that have retained immune memory against these tumor clones.
About ImmunityBio
ImmunityBio is a vertically-integrated commercial stage biotechnology company developing next-generation therapies that bolster the natural immune system to defeat cancers and infectious diseases. The Company’s range of immunotherapy and cell therapy platforms, alone and together, act to drive and sustain an immune response with the goal of creating durable and safe protection against disease. Designated an FDA Breakthrough Therapy, ANKTIVA is the first FDA-approved immunotherapy for non-muscle invasive bladder cancer CIS that activates NK cells, T cells, and memory T cells for a long-duration response. The Company is applying its science and platforms to treating cancers, including the development of potential cancer vaccines, as well as developing immunotherapies and cell therapies that we believe sharply reduce or eliminate the need for standard high-dose chemotherapy. These platforms and their associated product candidates are designed to be more effective, accessible, and easily administered than current standards of care in oncology and infectious diseases. For more information, visit ImmunityBio.com (Founder’s Vision) and connect with us on X (Twitter), Facebook, LinkedIn, and Instagram.
Forward Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, such as statements regarding efficacy and safety data from the ongoing QUILT-106 clinical study (NCT06334991) evaluating an off-the-shelf CD19 chimeric antigen receptor natural killer cell therapy (CAR-NK) in combination with rituximab for patients with Waldenström non-Hodgkin lymphoma, a rare B-cell malignancy and potential implications therefrom, potential regulatory pathways and approval requests and submissions, the regulatory review process and timing thereof, potential benefits to patients, potential treatment outcomes for patients, the described mechanism of action and results and contributions therefrom, information regarding clinical trials, including potential trial design and timing, potential future uses and applications of infusing CD-19 CAR NK cells with rituximab, and ImmunityBio’s approved product and investigational agents as compared to existing treatment options, among others. The clinical trial referenced in this release is ongoing, and the data described are interim, subject to change, and based on data available as of a specified data cutoff date. No conclusions regarding efficacy, durability of response, comparative benefit, or long-term clinical outcomes can be drawn at this time. As patient enrollment continues and additional follow-up is obtained, the reported response duration, safety profile, and other clinical outcomes may change materially. There can be no assurance that the interim results will be predictive of final study results or that additional data will confirm or support these observations.
Statements in this presentation that are not statements of historical fact are considered forward-looking statements, which are usually identified by the use of words such as “anticipates,” “believes,” “continues,” “goal,” “could,” “estimates,” “scheduled,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “indicate,” “projects,” “is,” “seeks,” “should,” “will,” “strategy,” and variations of such words or similar expressions. Statements of past performance, efforts, or results of our preclinical and clinical trials, about which inferences or assumptions may be made, can also be forward-looking statements and are not indicative of future performance or results. Forward-looking statements are neither forecasts, promises nor guarantees, and are based on the current beliefs of ImmunityBio’s management as well as assumptions made by and information currently available to ImmunityBio. Such information may be limited or incomplete, and ImmunityBio’s statements should not be read to indicate that it has conducted a thorough inquiry into, or review of, all potentially available relevant information. Such statements reflect the current views of ImmunityBio with respect to future events and are subject to known and unknown risks, including business, regulatory, economic and competitive risks, uncertainties, contingencies and assumptions about ImmunityBio, including, without limitation, the preliminary and evolving nature of interim clinical data; potential safety, tolerability, or dosing issues that may emerge with longer follow-up or in additional patients; variability in patient response; limitations related to study design, size, and duration; challenges in clinical trial conduct, enrollment, and retention; the timing and outcome of regulatory interactions; the possibility that the investigational therapy may not demonstrate sufficient safety or efficacy to support further development or regulatory approval; ImmunityBio’s ability to obtain additional financing to fund its operations and complete the development and commercialization of its various product candidates; and ImmunityBio’s ability to obtain, maintain, protect and enforce patent protection and other proprietary rights for its product candidates and technologies.
More details about these and other risks that may impact ImmunityBio’s business are described under the heading “Risk Factors” in the Company’s Form 10-K filed with the
View source version on businesswire.com: https://www.businesswire.com/news/home/20260116046721/en/
ImmunityBio Contacts:
Investors
Hemanth Ramaprakash, PhD, MBA
ImmunityBio, Inc.
+1 858-746-9289
Hemanth.Ramaprakash@ImmunityBio.com
Media
Sarah Singleton
ImmunityBio, Inc.
+1 415-290-8045
Sarah.Singleton@ImmunityBio.com
Source: ImmunityBio, Inc.







