InflaRx Provides Update on Phase 3 Data Analyses for Vilobelimab in Pyoderma Gangrenosum
Rhea-AI Summary
InflaRx (Nasdaq: IFRX) provided updated analyses from its terminated Phase 3 study of vilobelimab in ulcerative pyoderma gangrenosum (PG). The trial was stopped early for futility after enrolling 54 patients (30 completed 6 months). The prespecified primary endpoint (complete target ulcer closure on two consecutive visits) favored vilobelimab 20.8% vs placebo 16.7% (p=NS). Post-hoc analyses showed statistically significant reductions in target ulcer volume: MMRM percent change overall -45.4% (p=0.0428) and weekly differences from Week 14 to 26 (e.g., Week 26 -63.2%, p=0.0122); ANCOVAs for volume and area were p=0.0111 and p=0.0072. Safety was generally well tolerated with similar serious related TEAEs (6.3% vs 4.5%). InflaRx plans FDA discussions and expects future PG work would require a partner.
Positive
- MMRM: target ulcer volume -45.4% overall (Weeks 2–26, p=0.0428)
- Significant weekly volume differences Week 14–26 (Week 26 -63.2%, p=0.0122)
- ANCOVA: mean percent change in volume (p=0.0111) and area (p=0.0072)
- Safety: serious related TEAEs similar (6.3% vilobelimab vs 4.5% placebo)
Negative
- Primary endpoint not met: complete target ulcer closure 20.8% vs 16.7% (p=NS)
- IDMC recommended early termination for futility after 54 patients enrolled
- Small sample and limited 6‑month completers (30 patients completed 6 months)
- InflaRx will not allocate significant resources; future PG work requires a partner
News Market Reaction
On the day this news was published, IFRX declined NaN%, reflecting a moderate negative market reaction. Argus tracked a trough of -20.8% from its starting point during tracking. Our momentum scanner triggered 4 alerts that day, indicating moderate trading interest and price volatility.
Data tracked by StockTitan Argus on the day of publication.
Key Figures
Market Reality Check
Peers on Argus
IFRX declined 7.27% while close biotech peers were mixed: STTK rose 7.03%, SRZN, HLVX, MGNX and XBIT fell between 0.48% and 1.76%, suggesting a stock-specific reaction.
Historical Context
| Date | Event | Sentiment | Move | Catalyst |
|---|---|---|---|---|
| Dec 11 | Drug naming update | Positive | -2.9% | WHO assigned international nonproprietary name izicopan for INF904. |
| Nov 10 | Positive trial data | Positive | +28.5% | Positive Phase 2a INF904 data in HS and CSU with symptom reductions. |
| Nov 07 | Data readout preview | Positive | +2.5% | Announcement of upcoming Phase 2a INF904 topline data and webcast. |
| Oct 21 | Conference participation | Positive | +1.5% | Planned participation in Guggenheim healthcare innovation conference and meetings. |
| Sep 12 | Nasdaq compliance | Positive | -14.3% | Regained compliance with Nasdaq minimum bid price requirement. |
Recent news often triggered sharp moves, with both positive catalysts and compliance milestones sometimes followed by downside, indicating uneven news-to-price alignment.
Over the last six months, InflaRx has highlighted regulatory and clinical milestones across its C5a/C5aR programs. On Sep 12, it regained Nasdaq minimum bid compliance, yet shares fell 14.29%. Subsequent conference participation on Oct 21 and a Phase 2a INF904 data preview on Nov 7 each saw modest gains. Strong INF904 efficacy data on Nov 10 drove a 28.46% jump, while the INN naming of izicopan on Dec 11 coincided with a small decline. Today’s PG Phase 3 analysis update fits a pattern of mixed market responses to clinical news.
Market Pulse Summary
This announcement revisits the vilobelimab Phase 3 PG trial, which had been stopped for futility, by providing detailed post-hoc analyses showing ulcer volume reductions, remission trends, and DLQI improvements favoring treatment. The company plans FDA discussions on alternative endpoints and indicates future PG work would likely require a partner while it prioritizes izicopan (INF904). Investors may focus on durability of these signals, regulatory feedback, and how resources balance between vilobelimab and INF904. Key metrics include the reported 20.8% remission and MMRM volume changes.
Key Terms
pyoderma gangrenosum medical
post-hoc analyses technical
intent-to-treat technical
Dermatology Life Quality Index (DLQI) medical
treatment-emergent adverse events (TEAEs) medical
mixed model repeated measures technical
analysis of covariance technical
last observation carried forward (LOCF) technical
AI-generated analysis. Not financial advice.
- While the Phase 3 trial of vilobelimab in ulcerative pyoderma gangrenosum (PG) was terminated earlier this year due to futility regarding its prespecified primary endpoint (as previously disclosed), subsequent post-hoc analyses suggest a positive trend in favor of vilobelimab, with signals indicating a potentially consistent treatment effect
- InflaRx anticipates meeting with the FDA to determine a potential development path forward in PG, which the Company anticipates would only be conducted in collaboration with a partner
JENA, Germany, Dec. 30, 2025 (GLOBE NEWSWIRE) -- InflaRx N.V. (Nasdaq: IFRX), a biopharmaceutical company pioneering anti-inflammatory therapeutics by targeting the complement system, today outlined multiple data analyses of the Phase 3 study for vilobelimab in pyoderma gangrenosum (PG), which was terminated earlier this year after an Independent Data Monitoring Committee (IDMC) recommended the trial be stopped early due to futility. The analyses disclosed today include the primary intent-to-treat analysis and several post-hoc analyses on the 54 patients enrolled in the trial at the time of study termination.
Prof. Niels C. Riedemann, Chief Executive Officer and Founder of InflaRx, said: “Our Phase 3 study was the first randomized placebo-controlled study in pyoderma gangrenosum using complete target ulcer closure on two consecutive visits as a stringent primary clinical endpoint, which has not been tested before in this rare disease. Our in-depth data analysis reveals signals of efficacy, particularly regarding ulcer volume reduction, which further supports the potential role of the C5a/C5aR pathway in certain neutrophilic skin diseases, such as PG. Depending on the outcome of our anticipated FDA interactions, our results may provide an opportunity to advance development in collaboration with a partner, which we may pursue in the future.”
Results of Phase 3 data analysis
The Phase 3 study had recruited a total of 54 patients at the time the interim analysis was conducted, including 30 patients who had completed 6 months of treatment. The primary clinical endpoint of complete target ulcer closure on two consecutive visits showed a difference in favor of vilobelimab over placebo of
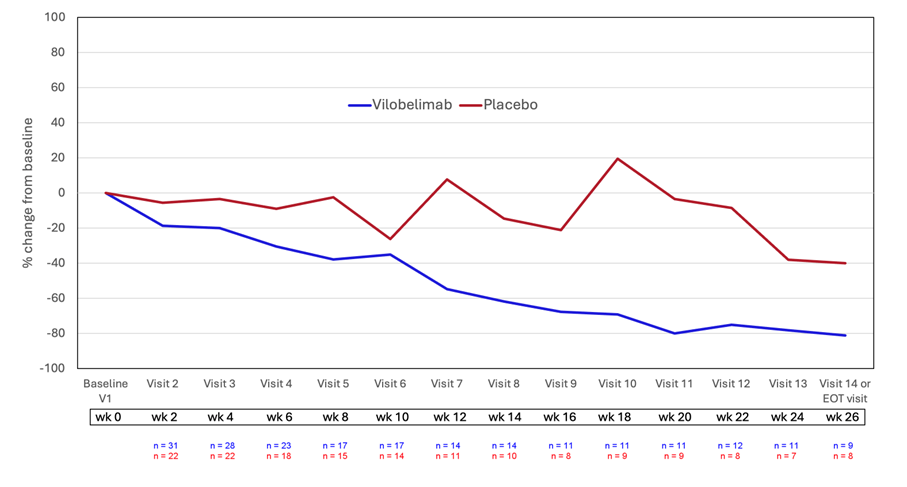
In addition, further post-hoc analyses showed that there is an overall treatment effect with vilobelimab when compared to placebo. These include an MMRM (mixed model repeated measures) for percent change in target ulcer volume, which showed an average effect over all visits in favor of vilobelimab over placebo (-
In addition, ANCOVAs (analyses of covariance) for mean of percentage changes from baseline in volume and area from Week 12 until Week 26 were also in favor of vilobelimab, including mean of percentage change from baseline in volume (p=0.0111) and area (p=0.0072). These analyses suggest that treatment longer than 26 weeks with vilobelimab may provide improved treatment outcomes in this difficult-to-treat ulcerative PG population.
Figure 1: Ulcer volume mean percent change from baseline, vilobelimab versus placebo
Alex G. Ortega Loayza, MD, MCR, CWSP, Professor and Interim Chair, Department of Dermatology, Oregon Health and Science University, said: “I am encouraged by the signals of efficacy observed from the Phase 3 post-hoc analyses of vilobelimab in pyoderma gangrenosum. The role of targeting C5a/C5aR aligns with prior mechanistic work supporting its use for this devastating inflammatory dermatological disease, which has no FDA-approved therapy. Given the significant unmet need and lack of approved therapies, I am hopeful these findings will motivate further investigation of this targeted approach.”
Benjamin Kaffenberger, MD, Associate Professor, Dermatology, The Ohio State University Wexner Medical Center, said: “Pyoderma gangrenosum remains a difficult-to-treat rare disease with high unmet medical need. The data for vilobelimab suggest an overall treatment effect, even if the primary endpoint may not have been achieved within the six-month timeframe required in this first-of-its-kind Phase 3 trial. Unfortunately, there is not a successful precedent for conducting a pivotal Phase 3 placebo-controlled study in PG, and I believe the futility leading to early discontinuation relates to challenges in trial design rather than lack of efficacy of the therapy. I believe that blocking C5a/C5aR in this neutrophilic disease continues to make scientific and clinical sense and that there is a strong justification to move forward.”
As next steps, InflaRx anticipates meeting with the FDA to discuss a potential path forward for vilobelimab in PG, including the use of alternative endpoints that could be utilized for potential future clinical studies. At this time, in an effort to prioritize izicopan (INF904) development, InflaRx does not expect to deploy significant resources towards future vilobelimab development in PG on its own and will instead consider doing so in collaboration with a partner.
About vilobelimab
Vilobelimab is a first-in-class monoclonal anti-human complement factor C5a antibody, which highly and effectively blocks the biological activity of C5a and demonstrates high selectivity towards its target in human blood. Thus, vilobelimab leaves the formation of the membrane attack complex (C5b-9) intact as an important defense mechanism of the innate immune system, which is not the case for molecules blocking C5. In pre-clinical studies, vilobelimab has been shown to control the inflammatory response-driven tissue and organ damage by specifically blocking C5a as a key “amplifier” of this response.
About InflaRx N.V.
InflaRx (Nasdaq: IFRX) is a biopharmaceutical company pioneering anti-inflammatory therapeutics by applying its proprietary anti-C5a and anti-C5aR technologies to discover, develop and commercialize highly potent and specific inhibitors of the complement activation factor C5a and its receptor, C5aR. C5a is a powerful inflammatory mediator involved in the progression of a wide variety of inflammatory diseases. InflaRx has developed vilobelimab, a novel, intravenously delivered, first-in-class, anti-C5a monoclonal antibody that selectively binds to free C5a and has demonstrated disease-modifying clinical activity and tolerability in multiple clinical studies. InflaRx is also developing izicopan (INF904), an orally administered small molecule inhibitor of C5a-induced signaling via the C5a receptor.
InflaRx was founded in 2007, and the group has offices and subsidiaries in Jena and Munich, Germany, as well as Ann Arbor, MI, USA. For further information, please visit www.inflarx.de. InflaRx GmbH (Germany) and InflaRx Pharmaceuticals Inc. (USA) are wholly owned subsidiaries of InflaRx N.V. (together, InflaRx).
| Contacts: | |
| InflaRx N.V. | MC Services AG |
| Jan Medina, CFA Vice President, Head of Investor Relations Email: IR@inflarx.de | Katja Arnold, Laurie Doyle, Dr. Regina Lutz Email: inflarx@mc-services.eu Europe: +49 89-210 2280 U.S.: +1-339-832-0752 |
FORWARD-LOOKING STATEMENTS
This press release contains forward-looking statements. All statements other than statements of historical fact are forward-looking statements, which are often indicated by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “estimate,” “believe,” “predict,” “potential” or “continue,” among others. Forward-looking statements appear in a number of places throughout this press release and may include statements regarding our intentions, beliefs, projections, outlook, analyses and current expectations concerning, among other things: the receptiveness of izicopan as a treatment for HS and CSU by patients and hospitals and related treatment recommendations by medical/healthcare institutes and other third-party organizations; our ability to successfully secure distribution channels and commercialize GOHIBIC (vilobelimab) as a treatment for COVID-19 patients and our ability to positively influence treatment recommendations by U.S. and European hospitals, guideline bodies and other third-party organizations; our expectations regarding the size of the patient populations for, market opportunity for, coverage and reimbursement for, estimated returns and return accruals for, and clinical utility of GOHIBIC (vilobelimab) in its approved or authorized indication or for vilobelimab and any other product candidates, under the Emergency Use Authorization and in the future if approved for commercial use in the United States, Europe or elsewhere; our ability to successfully implement The InflaRx Commitment Program, the success of our future clinical trials for vilobelimab’s treatment of debilitating or life-threatening inflammatory indications, including acute respiratory distress syndrome and other indications, and any other product candidates, including izicopan, and whether such clinical results will reflect results seen in previously conducted pre-clinical studies and clinical trials; the timing, progress and results of pre-clinical studies and clinical trials of vilobelimab, izicopan and any other product candidates, including for the development of vilobelimab in several indications, including to obtain full approval of GOHIBIC (vilobelimab) for COVID-19 and other virally induced ARDS, to treat HS and CSU, and statements regarding the timing of initiation and completion of studies or trials and related preparatory work, the period during which the results of the trials will become available, the costs of such trials and our research and development programs generally; our interactions with and the receptiveness and approval by regulators regarding the results of clinical trials and potential regulatory approval or authorization pathways, including our biologics license application submission for GOHIBIC (vilobelimab); the timing and outcome of any discussions or submission of filings for regulatory approval or authorization of vilobelimab, izicopan or any other product candidate, and the timing of and our ability to obtain and maintain full regulatory approval, the EUA and/or market authorization of vilobelimab or GOHIBIC (vilobelimab) for any indication; our ability to leverage our proprietary anti-C5a and anti-C5aR technologies to discover and develop therapies to treat complement-mediated autoimmune and inflammatory diseases; our ability to protect, maintain and enforce our intellectual property protection for vilobelimab, izicopan and any other product candidates, and the scope of such protection; whether the U.S. Food and Drug Administration, the European Medicines Agency or any comparable foreign regulatory authority will accept or agree with the number, design, size, conduct or implementation of our clinical trials, including any proposed primary or secondary endpoints for such trials; the success of our future clinical trials for vilobelimab, izicopan and any other product candidates and whether such clinical results will reflect results seen in previously conducted pre-clinical studies and clinical trials; our expectations regarding the size of the patient populations for, the market opportunity for, the medical need for and clinical utility of vilobelimab, izicopan or any other product candidates, if approved or authorized for commercial use; our manufacturing capabilities and strategy, including the scalability and cost of our manufacturing methods and processes and the optimization of our manufacturing methods and processes, and our ability to continue to rely on our existing third-party manufacturers and our ability to engage additional third-party manufacturers for our planned future clinical trials and for commercial supply of vilobelimab and for the finished product GOHIBIC (vilobelimab) in the United States and Europe; our estimates of our expenses, ongoing losses, future revenue, capital requirements and our needs for or ability to obtain additional financing; our expectations regarding the scope of any approved indication for vilobelimab; our ability to defend against liability claims resulting from the testing of our product candidates in the clinic or, if approved or authorized, any commercial sales; if any of our product candidates obtain regulatory approval or authorization, our ability to comply with and satisfy ongoing drug regulatory obligations and continued regulatory overview; our ability to comply with enacted and future legislation in seeking marketing approval or authorization and commercialization; our future growth and ability to compete, which depends on our retaining key personnel and recruiting additional qualified personnel; our competitive position and the development of and projections relating to our competitors in the development of C5a and C5aR inhibitors and other therapeutic products being developed in similar medical conditions in which vilobelimab, izicopan or any other of our product candidates is being developed or our industry; and the risks, uncertainties and other factors described under the heading “Risk Factors” in our periodic filings with the U.S. Securities and Exchange Commission. These statements speak only as of the date of this press release and involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Given these risks, uncertainties and other factors, you should not place undue reliance on these forward-looking statements, and we assume no obligation to update these forward-looking statements, even if new information becomes available in the future, except as required by law.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/5dbcac2a-4ce3-4744-b719-8548e705aef5








