Pasithea Therapeutics Announces Completion of Cohort 7 in Ongoing Phase 1 Trial of PAS-004 in Advanced Cancer Patients, with Positive Safety, Pharmacokinetic (PK), and Pharmacodynamic (PD) Data
Rhea-AI Summary
Pasithea Therapeutics (Nasdaq: KTTA) announced completion of Cohort 7 (37 mg) in its Phase 1 PAS-004 trial on Nov 24, 2025, reporting positive safety, PK, and PD data.
Key findings: zero treatment-related adverse events during the DLT period; Safety Review Committee cleared escalation to Cohort 8 (45 mg); PK showed linear, dose-proportional behavior with AUC 6,690 ng·h/mL, Cmax 313 ng/mL, Cmin 260 ng/mL, and Cmax/Cmin ratio <2; PD showed ~80% pERK inhibition near Cmax and >60% pERK inhibition at Cmin (24 hr), supporting continuous MAPK pathway suppression over a 24-hour dosing cycle.
Positive
- Zero treatment-related adverse events observed during Cohort 7 DLT period
- Safety Review Committee recommended escalation to Cohort 8 (45 mg)
- AUC 6,690 ng·h/mL at steady state (Cohort 7)
- Cmax 313 ng/mL and Cmin 260 ng/mL with Cmax/Cmin <2
- ~80% pERK inhibition near Cmax and >60% at Cmin, indicating 24-hour pathway suppression
Negative
- None.
Insights
Phase 1 Cohort 7 shows clean short‑term safety, linear PK, and continuous MAPK suppression at 24 hours.
PAS-004 in Cohort 7 (37mg capsules) reported no treatment‑related adverse events during the DLT window and no DLTs, supporting a safe short‑term tolerability profile. The PK profile was linear and dose‑proportional with reported AUC of 6,690 ng*h/mL, Cmax of 313 ng/mL and Cmin of 260 ng/mL, and a Cmax/Cmin ratio <2, indicating limited peak‑to‑trough fluctuation.
PD measures show target engagement consistent with sustained pathway suppression: mean inhibition of pERK ~
The Safety Review Committee recommended escalation to Cohort 8 (45mg capsules), which provides a clear near‑term milestone to monitor. Watch for emerging safety signals beyond the DLT window, confirmed PD at the higher dose, and any changes in exposure metrics at
-- Zero Treatment Related Adverse Events observed during Cohort 7 (37mg capsules) DLT period –
--Cohort 7 Pharmacokinetic (PK) profile continues to demonstrate dose-proportionality and Cmax/Cmin ratio <2. Achieved exposures (AUC) of 6,690 ng·h/mL –
-- Pharmacodynamic (PD) data support continuous suppression of MAPK pathway throughout the 24-hour cycle –
-- Safety Review Committee recommended that trial escalate to the next dose level Cohort (Cohort 8, 45mg capsules) –
MIAMI, Nov. 24, 2025 (GLOBE NEWSWIRE) -- Pasithea Therapeutics Corp. (Nasdaq: KTTA) (“Pasithea” or the “Company”), a clinical-stage biotechnology company developing PAS-004, a next-generation macrocyclic oral MEK inhibitor for the treatment of neurofibromatosis type 1-associated plexiform neurofibromas (NF1-PN), today announced positive safety, PK and PD data from Cohort 7 (37mg capsules) in its ongoing first-in-human trial evaluating PAS-004 in patients with MAPK pathway-driven advanced solid tumors with a documented RAS, NF1 or RAF mutation, or in patients who have failed prior BRAF/MEK inhibition (NCT06299839).
Dr. Tiago Reis Marques, Chief Executive Officer of Pasithea commented, “We are highly encouraged by the initial safety data generated in Cohort 7 (37mg capsules), where zero treatment-related adverse events have been observed during the DLT period. Furthermore, PD data demonstrates the pharmacological profile we believe is necessary to achieve consistent pathway inhibition over the 24-hour dosing period, while avoiding both periods of excessive suppression and periods of insufficient target engagement. We believe this balanced profile will be critical for achieving clinical efficacy while minimizing the most commonly observed adverse events associated with MEK inhibitors. We believe PAS-004 is particularly well suited for the treatment of diseases involving the MAPK pathway that require chronic dosing over long periods of time, where sustained long-term pathway suppression at safe and well-tolerated doses is required.”
PAS-004 has demonstrated in Cohort 7 (37mg capsules):
Safety and Tolerability Results:
- PAS-004 was safe and well tolerated with no dose limiting toxicities (DLTs), and no treatment-related adverse events observed during the DLT period.
- After reviewing cohort 7 data, the Safety Review Committee recommended to proceed to Cohort 8, 45mg capsules, without modification.
Pharmacodynamics (PD) Results:
- At steady-state, individual patient plasma data showed PAS-004 inhibiting phosphorylated extracellular signal-regulated kinase (pERK) at a level of
80% near Cmax. - At steady-state, individual patient plasma data showed PAS-004 inhibiting pERK at a level above
60% at Cmin (24-hour postdose).
Pharmacokinetics (PK) Results:
- Linear PK and dose-proportionality.
- PK curve with Cmax/Cmin ratio <2.
- AUC: 6,690 ng*h/mL; Cmax: 313 ng/mL; Cmin: 260 ng/mL.
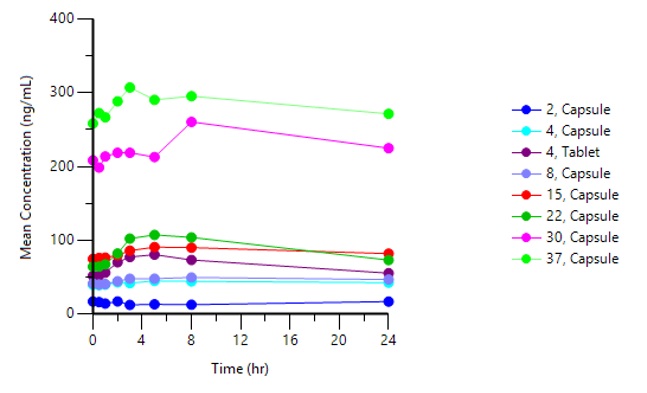
Graph 1 below represents the complete PAS-004 dose escalation curve at steady state in our ongoing Phase 1 trial in advanced cancer patients:
Graph 1:
About Pasithea Therapeutics Corp.
Pasithea is a clinical-stage biotechnology company primarily focused on the research and development of its lead drug candidate, PAS-004, a next-generation macrocyclic MEK inhibitor intended for the treatment of RASopathies, MAPK pathway-driven tumors, and other diseases. The Company is currently testing PAS-004 in a Phase 1 clinical trial in advanced cancer patients (NCT06299839), and a Phase 1/1b clinical trial in adult patients with neurofibromatosis type 1 (NF1)-associated plexiform neurofibromas (NCT06961565).
Forward Looking Statements
This press release contains statements that constitute “forward-looking statements” made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These forward-looking statements include statements regarding the Company’s ongoing Phase 1 clinical trial of PAS-004 in advanced cancer patients, the Company’s ongoing Phase 1/1b clinical trial of PAS-004 in adult NF1 patients, and the safety, tolerability, pharmacokinetic (PK), pharmacodynamics (PD) and preliminary efficacy of PAS-004, as well as all other statements, other than statements of historical fact, regarding the Company’s current views and assumptions with respect to future events regarding its business, as well as other statements with respect to the Company’s plans, assumptions, expectations, beliefs and objectives, the success of the Company’s current and future business strategies, product development, pre-clinical studies, clinical studies, clinical and regulatory timelines, market opportunity, competitive position, business strategies, potential growth and financing opportunities and other statements that are predictive in nature. Forward-looking statements are subject to numerous conditions, many of which are beyond the control of the Company. While the Company believes these forward-looking statements are reasonable, undue reliance should not be placed on any such forward-looking statements, which are based on information available to the Company on the date of this release. These forward-looking statements are based upon current estimates and assumptions and are subject to various risks and uncertainties, including risks that future clinical trial results may not match results observed to date, may be negative or ambiguous, or may not reach the level of statistical significance required for regulatory approval, as well as other factors set forth in the Company’s most recent Annual Report on Form 10-K, Quarterly Reports on Form 10-Q and other filings made with the U.S. Securities and Exchange Commission. Thus, actual results could be materially different. The Company undertakes no obligation to update these statements whether as a result of new information, future events or otherwise, after the date of this release, except as required by law.
Pasithea Therapeutics Contact
Patrick Gaynes
Corporate Communications
pgaynes@pasithea.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/0262b563-9c20-40a8-bad3-e207fd8943a2








