Pasithea Therapeutics Announces Positive PAS-004 Tablet Pharmacokinetic (PK) Data in Ongoing Phase 1/1b Trial in Adult NF1 Patients
Rhea-AI Summary
Pasithea Therapeutics (Nasdaq: KTTA) reported positive tablet pharmacokinetic (PK) data from its ongoing Phase 1/1b study of PAS-004 in adult NF1 patients on Nov 21, 2025. Key findings: linear, dose-proportional PK across 4mg and 8mg tablets; steady‑state Cmax/Cmin <2 with Cmax and Cmin above the cellular IC50; and a long half‑life (~57 hours). Cohort PK: 4mg AUC 1,120 ng·h/mL, Cmax 58.1 ng/mL, Cmin 37.6 ng/mL; 8mg AUC 2,290 ng·h/mL, Cmax 118 ng/mL, Cmin 75.4 ng/mL. Tablet exposures were ~3x higher (dose‑normalized) than capsule, with the 8mg tablet AUC/Cmax slightly exceeding the 22mg capsule and showing reduced interpatient variability and similar Tmax.
Positive
- Linear, dose-proportional PK across 4mg and 8mg tablets
- Steady‑state Cmax/Cmin <2 with levels above cellular IC50
- Long half‑life of ~57 hours
- 4mg AUC 1,120 ng·h/mL; 8mg AUC 2,290 ng·h/mL
- Tablet exposures ~3x higher dose‑normalized vs capsule
- 8mg tablet AUC/Cmax slightly greater than 22mg capsule
Negative
- None.
Insights
PAS-004 tablet shows dose-proportional, higher and more predictable exposure versus capsule; data may simplify dosing and development.
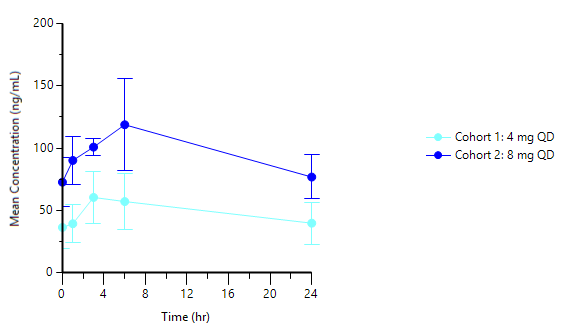
PAS-004 tablets at 4mg and 8mg produced linear, dose-proportional PK with a steady‑state Cmax/Cmin ratio <2 and a reported half‑life of ~57 hours. Cohort metrics include AUC 1,120 ng·h/mL, Cmax 58.1 ng/mL, Cmin 37.6 ng/mL for 4mg and AUC 2,290 ng·h/mL, Cmax 118 ng/mL, Cmin 75.4 ng/mL for 8mg; both Cmax and Cmin exceeded the stated IC50 from the cellular assay.
The tablet gives roughly 3-fold higher dose‑normalized exposure than the capsule, with the 8mg tablet achieving AUC and Cmax slightly above the 22mg capsule and showing reduced inter‑patient variability and similar Tmax. These facts imply formulation-driven bioavailability improvements that could lower required dose and tighten exposure margins, but clinical safety, tolerability, and efficacy outcomes still determine actual benefit.
Monitor forthcoming safety and efficacy readouts from the ongoing Phase
-- Tablet PK exposure increases proportionally with an increase in dose --
-- More favorable PK properties in tablets enable a lower dose to achieve the same exposure as the capsule formulation, with improved predictability and reduced variability --
-- Tablet steady state showing Cmax/Cmin ratio <2 --
MIAMI, Nov. 21, 2025 (GLOBE NEWSWIRE) -- Pasithea Therapeutics Corp. (Nasdaq: KTTA) (“Pasithea” or the “Company”), a clinical-stage biotechnology company developing PAS-004, a next-generation macrocyclic oral MEK inhibitor for the treatment of neurofibromatosis type 1-associated plexiform neurofibromas (NF1-PN), today announced positive tablet PK data from ongoing Phase 1/1b open-label study evaluating PAS-004 in adult patients with neurofibromatosis type 1 (NF1) with symptomatic and inoperable, incompletely resected, or recurrent plexiform neurofibromas (NCT06961565).
Pharmacokinetics (PK)
PAS-004 has demonstrated in the tablet formulation (4mg and 8mg cohorts):
- Linear PK and dose-proportionality
- PK curve with Cmax/Cmin ratio <2, with Cmax and Cmin above the IC50 (half-maximal inhibitory concentration) from our cellular assay
- Long half-life (~57 hours)
- Cohort 1 (4mg tablet) has demonstrated:
- AUC: 1,120 ng·h/mL
- Cmax: 58.1 ng/mL
Cmin: 37.6 ng/mL
- AUC: 1,120 ng·h/mL
- Cohort 2 (8mg tablet) has demonstrated:
- AUC: 2,290 ng·h/mL
- Cmax: 118 ng/mL
- Cmin: 75.4 ng/mL
- AUC: 2,290 ng·h/mL
Dose normalized exposures following once daily administration of PAS-004 tablets were approximately 3-fold higher than those following administration with the capsule formulation, resulting in the 8mg tablet area under the curve (AUC) and Cmax being slightly greater than those of the 22mg capsule. The tablet formulation has demonstrated less patient variability and a similar Tmax range when compared to the capsule formulation. This is consistent with the pre-clinical evaluation of the two formulations in the dog toxicology studies.
Graph 1 below represents the tablet PK curve at steady state for the 4mg and 8mg doses and Graph 2 below represents the 8mg tablet PK curve at steady state as compared to 22mg capsule dose at steady state from our ongoing Phase 1 trial in advanced cancer patients:
Graph 1:
Graph 2:

About Pasithea Therapeutics Corp.
Pasithea is a clinical-stage biotechnology company primarily focused on the research and development of its lead drug candidate, PAS-004, a next-generation macrocyclic MEK inhibitor intended for the treatment of RASopathies, MAPK pathway-driven tumors, and other diseases. The Company is currently testing PAS-004 in a Phase 1 clinical trial in advanced cancer patients (NCT06299839), and a Phase 1/1b clinical trial in adult patients with neurofibromatosis type 1 (NF1)-associated plexiform neurofibromas (NCT06961565).
Forward Looking Statements
This press release contains statements that constitute “forward-looking statements” made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These forward-looking statements include statements regarding the Company’s ongoing Phase 1 clinical trial of PAS-004 in advanced cancer patients, the Company’s ongoing Phase 1/1b clinical trial of PAS-004 in adult NF1 patients, and the safety, tolerability, pharmacokinetic (PK), pharmacodynamics (PD) and preliminary efficacy of PAS-004, as well as all other statements, other than statements of historical fact, regarding the Company’s current views and assumptions with respect to future events regarding its business, as well as other statements with respect to the Company’s plans, assumptions, expectations, beliefs and objectives, the success of the Company’s current and future business strategies, product development, pre-clinical studies, clinical studies, clinical and regulatory timelines, market opportunity, competitive position, business strategies, potential growth and financing opportunities and other statements that are predictive in nature. Forward-looking statements are subject to numerous conditions, many of which are beyond the control of the Company. While the Company believes these forward-looking statements are reasonable, undue reliance should not be placed on any such forward-looking statements, which are based on information available to the Company on the date of this release. These forward-looking statements are based upon current estimates and assumptions and are subject to various risks and uncertainties, including risks that future clinical trial results may not match results observed to date, may be negative or ambiguous, or may not reach the level of statistical significance required for regulatory approval, as well as other factors set forth in the Company’s most recent Annual Report on Form 10-K, Quarterly Reports on Form 10-Q and other filings made with the U.S. Securities and Exchange Commission. Thus, actual results could be materially different. The Company undertakes no obligation to update these statements whether as a result of new information, future events or otherwise, after the date of this release, except as required by law.
Pasithea Therapeutics Contact
Patrick Gaynes
Corporate Communications
pgaynes@pasithea.com
Graphs accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/456983e6-8204-4553-b5a5-972eabc348d5
https://www.globenewswire.com/NewsRoom/AttachmentNg/ed3ed272-0b62-4549-8ada-a84eedc6ce66








