Labcorp Expands Sexually Transmitted Infection (STI) Test Offerings with Rapid Syphilis Test
Rhea-AI Summary
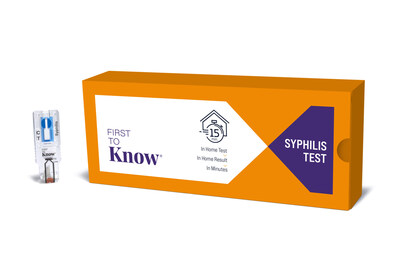
Labcorp (NYSE: LH) has expanded its STI testing portfolio with the First to Know® Syphilis Test, the first FDA-authorized over-the-counter blood test for syphilis. As the exclusive distributor for NOWDiagnostics (NOWDx) in the U.S., Labcorp will offer this rapid test to healthcare providers nationwide by the end of 2024 and to patients through Labcorp OnDemand in 2025.
The test provides results in 15 minutes using a single drop of blood from a fingerstick. This expansion aims to combat the rising syphilis cases in the U.S., which have increased by 80% between 2018 and 2022, reaching over 207,000 reported cases in 2022. Labcorp's syphilis testing volume has more than doubled in the past decade, exceeding 5.5 million tests annually.
Positive
- Exclusive distribution agreement with NOWDiagnostics for the First to Know® Syphilis Test
- Expansion of STI testing portfolio with FDA-authorized rapid syphilis test
- Planned availability to healthcare providers by end of 2024 and patients through Labcorp OnDemand in 2025
- Syphilis testing volume has more than doubled over the past decade, exceeding 5.5 million tests annually
Negative
- None.
News Market Reaction 1 Alert
On the day this news was published, LH declined 0.32%, reflecting a mild negative market reaction.
Data tracked by StockTitan Argus on the day of publication.
Labcorp to serve as the exclusive NOWDiagnostics (NOWDx) distributor for clinical testing in the
Labcorp will serve as the exclusive distributor of the First to Know Syphilis Test to providers in healthcare settings nationwide, through an agreement with over-the-counter and point-of-care diagnostic test developer NOWDiagnostics (NOWDx). This new offering reflects Labcorp's commitment to expanding provider and patient access to fast and reliable syphilis tests in an effort to help combat the syphilis epidemic.
"The recent rise in syphilis cases highlights a critical gap in testing and treatment across the country," said Dr. Brian Caveney, Labcorp's chief medical and scientific officer. "This test is another tool in a provider's arsenal as they face this serious public health emergency. Our agreement with NOWDx reflects our commitment to providing healthcare providers and patients across the country with access to tests that deliver fast and reliable results that empower them to make informed decisions about their health."
Labcorp will leverage its scale as one of the largest diagnostics laboratories in the world to facilitate widespread availability of the First to Know Syphilis Test, which provides a result in 15 minutes with as little as a single drop of blood collected through a fingerstick. Physicians can utilize the test in clinical settings or give it to a patient to conduct in the comfort of their own home. Labcorp plans to make the test available to providers by the end of 2024 and directly to patients through Labcorp OnDemand in 2025.
"Labcorp's strong infrastructure and commitment to advancing diagnostic technology and making testing widely accessible make it the ideal partner to help expand access to our tests and tackle critical public health issues, such as the rise in syphilis cases," said Robert Weigle, CEO of NOWDx. "Together, we aim to ensure our cutting-edge, user-friendly tests are within reach for more patients and healthcare providers, ultimately enhancing early identification and treatment outcomes."
As the FDA has noted, results from the First to Know Syphilis Test alone are not sufficient to diagnose syphilis infection and should be followed by additional testing to confirm a diagnosis of syphilis.
Labcorp currently offers treponemal and non-treponemal assays recommended by the Centers for Disease Control and Prevention (CDC) for the screening and diagnosis of syphilis. The company has seen syphilis testing volume more than double over the past decade, exceeding 5.5 million tests annually.
For more information, contact diagnosticsales@labcorp.com.
About Labcorp
Labcorp (NYSE: LH) is a global leader of innovative and comprehensive laboratory services that helps doctors, hospitals, pharmaceutical companies, researchers and patients make clear and confident decisions. We provide insights and advance science to improve health and improve lives through our unparalleled diagnostics and drug development laboratory capabilities. The company's more than 67,000 employees serve clients in approximately 100 countries, provided support for
About NOWDiagnostics (NOWDx)
NOWDx develops and manufactures over-the-counter (OTC) and point-of-care (POC) diagnostic tests. Its patented approach enables virtually any immunological assay to be accurately performed onsite in one step using a small amount of capillary blood, yielding results in minutes. With over 75 patents issued and pending, NOWDx's First To Know® and ADEXUSDx® product lines are available in markets worldwide. Founded in 2013, with headquarters and manufacturing in
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/labcorp-expands-sexually-transmitted-infection-sti-test-offerings-with-rapid-syphilis-test-302272890.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/labcorp-expands-sexually-transmitted-infection-sti-test-offerings-with-rapid-syphilis-test-302272890.html
SOURCE Labcorp