Lunai Bioworks Identifies Three Parkinson's Subtypes and Prioritized Drug Targets to Accelerate Proof-of-Concept Programs and Strategic Partnerships in a $13B Market
Rhea-AI Summary
Lunai Bioworks (NASDAQ: LNAI) announced identification of three clinically relevant Parkinson's disease subtypes and prioritized drug targets intended to accelerate proof-of-concept programs and strategic partnerships.
Analysis integrated longitudinal clinical and proteomic data from >650 participants and ~4,500 proteomic probes (median follow-up ≥2.5 years), linking subtypes to rapid progression, cognitive decline, and functional impairment. Lunai is initiating experimental validation, preclinical model development, and biomarker qualification while evaluating co-development opportunities in a market projected to exceed $13 billion by the 2030s.
Positive
- Three Parkinson's subtypes identified from subject-level analyses
- Data set: >650 participants and 4,500 proteomic probes
- Findings support biomarker-enriched patient selection and faster proof-of-concept
- Prioritized drug targets moved to experimental validation and preclinical development
Negative
- Target validation is preclinical and not yet clinically qualified
- Biomarker qualification and clinical utility remain unproven pending further studies
News Market Reaction
On the day this news was published, LNAI declined 8.70%, reflecting a notable negative market reaction. Argus tracked a peak move of +2.0% during that session. Argus tracked a trough of -23.6% from its starting point during tracking. Our momentum scanner triggered 14 alerts that day, indicating notable trading interest and price volatility. This price movement removed approximately $3M from the company's valuation, bringing the market cap to $32M at that time.
Data tracked by StockTitan Argus on the day of publication.
Key Figures
Market Reality Check
Peers on Argus
No peers from the Pharmaceutical Preparations sector appeared in the momentum scanner and no same-day peer headlines were provided, indicating the +10.4% move in LNAI looks stock-specific rather than sector-driven.
Historical Context
| Date | Event | Sentiment | Move | Catalyst |
|---|---|---|---|---|
| Nov 25 | Licensing LOI, oncology | Positive | -15.0% | Secured first LOI to license DC combination therapy after strong preclinical data. |
| Nov 05 | Preclinical cancer data | Positive | +40.3% | Reported complete regression of pancreatic tumors in humanized models with optimized DC therapy. |
| Oct 30 | AI neurotoxicity platform | Positive | -2.0% | Announced AI model with 0.94 AUROC for AChE inhibitor detection and zebrafish profiling plans. |
| Oct 16 | DMD biomarker study | Positive | -1.6% | Published large-scale serum proteomics study identifying prognostic biomarkers for DMD outcomes. |
| Oct 16 | Nasdaq compliance | Positive | -1.6% | Regained compliance with Nasdaq’s minimum bid requirement under Listing Rule 5550(a)(2). |
Positive scientific and platform news has often seen weak or negative next-day reactions, with only one clear alignment between upbeat news and a strong price gain.
Over the past few months, Lunai Bioworks has reported multiple scientific and strategic milestones. In Oct 2025, it published prognostic DMD biomarkers and separately announced regained compliance with Nasdaq’s minimum bid rule, both followed by modest negative reactions. Subsequent AI and neurotoxicity work in Oct 2025 and a landmark pancreatic cancer regression update on Nov 5 drew mixed trading, including a 40.29% jump. A later licensing LOI with complete tumor regression data on Nov 25 was met with a -14.96% move. Today’s Parkinson’s stratification news fits this pattern of substantial scientific progress with variable price responses.
Market Pulse Summary
The stock moved -8.7% in the session following this news. A negative reaction despite positive news would fit a recent pattern where strong scientific updates did not consistently support price strength. Past announcements, including licensing progress and biomarker discoveries, saw several negative next-day moves despite constructive content. With current volume often below the 3,035,709 20-day average, downside moves could be exaggerated by thin liquidity, and prior divergence between news quality and price shows sentiment has been cautious.
Key Terms
proteomic medical
proteomics medical
biomarker medical
companion diagnostics medical
phenotyping medical
longitudinal medical
precision therapeutics medical
companion diagnostics medical
AI-generated analysis. Not financial advice.
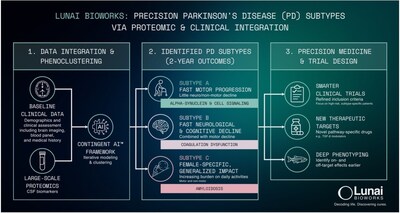
Using its proprietary Augusta Platform, Lunai Bioworks' wholly owned subsidiary BioSymetrics integrated large-scale proteomic data from the Parkinson's Progression Markers Initiative (PPMI), a landmark longitudinal study managed by The Michael J. Fox Foundation that tracks thousands of patients over time to identify biological markers of disease progression, with high-resolution clinical phenotyping to uncover subtypes associated with rapid progression, cognitive decline, and functional impairment. This analysis was recently highlighted in a recent article in Science Times.
The analysis integrated longitudinal clinical and proteomic data from more than 650 participants across 4,500 proteomic probes, tracked over multiple years (median ≥2.5 years, many ≥5 years). This scale enabled statistically robust identification of molecular signatures linked to rapid progression and worse outcomes, supporting biomarker-enriched patient selection, accelerated proof-of-concept, and higher probability of clinical and commercial success for subtype-specific therapeutics.
Subject-level analyses revealed three outcome-linked patient subtypes:
- Fast motor progression with limited non-motor involvement
- Rapid neurological and cognitive decline alongside motor worsening
- A female-enriched subtype with broad functional impairment
These findings create an actionable foundation for inclusion, enrichment, and endpoint strategies designed to improve trial success rates, time to proof-of-concept, and asset valuation.
Proteomic and pathway-level evaluation identified progression-linked targets and biomarker candidates that may support baseline stratification, progression monitoring, and treatment-response assessment. Lunai is initiating experimental validation of prioritized targets and advancing toward preclinical model development and biomarker qualification.
"As Parkinson's therapy development continues to struggle with high failure rates and slow progression signals, subtype-specific strategies can materially improve outcomes," said David Weinstein, CEO of Lunai Bioworks. "By linking clinical trajectories to biological pathways, we can design smarter trials, identify partnerable targets, and accelerate development timelines in a highly competitive market."
In parallel, Lunai Bioworks is evaluating co-development and partnering opportunities with biopharmaceutical companies to:
- Apply subtype-specific inclusion criteria to existing Parkinson's assets
- Co-develop biomarkers and companion diagnostics
- Translate pathway-level insights into first-in-class therapeutics
"Integrating molecular biology with clinical phenotyping gives us a mechanism for identifying precision targets that could reshape how Parkinson's therapies are developed," said Dr. Gabe Musso, Chief Scientific Officer of BioSymetrics. "This will enable faster validation and more efficient partnering strategies."
Parkinson's disease represents a
About Lunai Bioworks
Lunai Bioworks Inc. (NASDAQ: LNAI) is an AI-powered drug discovery and biodefense company pioneering safe and responsible generative biology. With proprietary neurotoxicity datasets, advanced machine learning, and a focus on dual-use risk management, Lunai Bioworks aims to redefine how artificial intelligence accelerates therapeutic innovation while safeguarding society from emerging threats. For more information, please visit: https://lunaibioworks.com.
Forward-Looking Statements
This press release contains forward-looking statements, including statements regarding potential clinical impact, therapeutic benefit, development timelines, partnering strategy, and commercial value. These statements are subject to risks and uncertainties that may cause actual results to differ materially. Lunai Bioworks undertakes no obligation to update forward-looking statements, except as required by law.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/lunai-bioworks-identifies-three-parkinsons-subtypes-and-prioritized-drug-targets-to-accelerate-proof-of-concept-programs-and-strategic-partnerships-in-a-13b-market-302636013.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/lunai-bioworks-identifies-three-parkinsons-subtypes-and-prioritized-drug-targets-to-accelerate-proof-of-concept-programs-and-strategic-partnerships-in-a-13b-market-302636013.html
SOURCE Lunai Bioworks Inc.