Medtronic announces results showing meaningful pain relief using DTM™ Spinal Cord Stimulation endurance therapy
Rhea-AI Summary
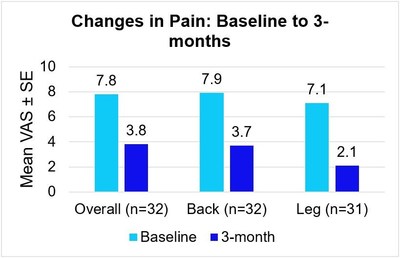
Medtronic plc (NYSE: MDT) announced positive results from a three-month study of its DTM SCS endurance therapy for chronic pain. The therapy showed a 3.9 cm reduction in overall pain, with patients experiencing a 4.3 cm decrease in back pain and a 5.0 cm drop in leg pain. 69% of patients reported less disability, and 75% expressed satisfaction with the treatment. The therapy enhances device longevity, offering 5.5-7.5 years on the Vanta™ stimulator and rapid recharge options on Intellis™. These findings underscore the therapy's effectiveness and potential for improving quality of life.
Positive
- 3.9 cm overall pain reduction reported by patients at 3 months.
- 69% of patients improved to a less disabled category per the Oswestry Disability Index.
- 75% of patients were satisfied with their therapy.
- Potential device longevity of 5.5-7.5 years with Vanta™ neurostimulator.
Negative
- None.
News Market Reaction
On the day this news was published, MDT declined 0.21%, reflecting a mild negative market reaction.
Data tracked by StockTitan Argus on the day of publication.
DTM™ SCS endurance therapy enables long-lasting recharge-free performance on Vanta™ and 5-minute recharge on Intellis™ neurostimulators
DUBLIN, Jan. 14, 2022 /PRNewswire/ -- Medtronic plc (NYSE: MDT), a global leader in healthcare technology, today announced three-month results from an on-label, prospective, multi-center study1 showing meaningful pain relief using DTM™ SCS endurance therapy, a modified, lower-energy variation of the company's Differential Target Multiplexed™ (DTM) Spinal Cord Stimulation (SCS) therapy for chronic overall, back or leg pain. At 3 months, patients treated with DTM SCS endurance therapy reported meaningful pain relief as measured by a 3.9 cm reduction in overall pain on the 10 cm Visual Analog Scale (VAS)2. Patients also reported an average 4.3 cm decrease in back pain, and an average 5.0 cm decrease in leg pain.
The Visual Analog Scale (VAS) is a widely used and accepted measure for pain intensity that captures patient-reported pain levels on a scale of 0-10. These 3-month results are consistent with those of a prior feasibility study3 and add to the body of DTM SCS evidence demonstrating that DTM SCS endurance therapy can provide effective pain relief along with additional quality of life and functional benefits for patients.
Additional 3-month data include:
69% of patients improved to a less disabled category as measured by the Oswestry Disability Index, with63% having minimal or moderate disability at 3-months compared to just16% at baseline75% of patients were very satisfied or somewhat satisfied with their therapy at 3-months
Modeling based on actual three-month data also shows that DTM SCS endurance therapy enables between 5.5-7.5 years device longevity when programmed on the Vanta™ recharge-free neurostimulator.4 For those in need of a rechargeable device, DTM SCS endurance therapy programmed on the Intellis™ rechargeable neurostimulator allows either rapid recharge (5 minutes per day) or recharges of approximately one hour every 12 days.4
"These 3-month results are highly encouraging, as they demonstrate that the DTM SCS endurance therapy may be able to provide effective pain relief while dosing at lower energy than other SCS waveforms. This may improve device longevity and offers validation of therapy for the patients who would need it," said Dr. Kasra Amirdelfan, director of Clinical Research at IPM Medical Group, Inc. and principal investigator on the DTM-LE Trial.
"Every patient with chronic pain has their own unique needs, and Medtronic is committed to offering solutions that personalize care for our patients," said Charlie Covert, vice president and general manager, Pain Therapies within the Neuromodulation business, which is part of the Neuroscience Portfolio at Medtronic. "For many, especially those who need or prefer a recharge-free SCS solution, our new DTM SCS endurance therapy offers meaningful clinical benefits by reducing pain, improving quality of life, and offering meaningful device longevity."
Enrolled patients will have additional follow-up assessments at 6 and 12 months.
These data were first shared at the 25th Annual North American Neuromodulation Society (NANS) Annual Meeting, January 13-15 2022.
About Medtronic
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Dublin, Ireland, is the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 90,000+ passionate people across 150 countries. Our technologies and therapies treat 70 health conditions and include cardiac devices, surgical robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for our world. In everything we do, we are engineering the extraordinary. For more information on Medtronic (NYSE: MDT), visit www.Medtronic.com and follow @Medtronic on Twitter and LinkedIn.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
References
1Using the Intellis™ with AdaptiveStim™ neuromodulation system
2Peacock, Provenzano, Fishman, et al. A Prospective Multi-Center Study of a Differential Target Multiplexed™ Stimulation Derivative in Therapy-Naive Patients: Primary Endpoint and 3-Month Outcomes. Poster presented at: North American Neuromodulation Society (NANS) Annual Meeting; Jan. 13-15, 2022; Orlando, FL.
3Fishman M, Hatheway J, Will A, et al. Utilization of Different Energy Profiles of Differential Target Multiplexed Spinal Cord Stimulation. American Society of Pain & Neuroscience (ASPN); July 22-25, 2021; Miami, FL. Poster.
4Provenzano, Amirdelfan, Grewal, et al. Modeling Energy Demands of a Reduced-Energy Derivative of Differential Target Multiplexed™ Stimulation on Rechargeable and Recharge-free Systems. Poster presented at North American Neuromodulation Society (NANS); January 13-15, 2022; Orlando, FL.
Contacts:
Jeff Trauring | Ryan Weispfenning |
Public Relations | Investor Relations |
+1-763-505-0159 | +1-763-505-4626 |
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/medtronic-announces-results-showing-meaningful-pain-relief-using-dtm-spinal-cord-stimulation-endurance-therapy-301461012.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/medtronic-announces-results-showing-meaningful-pain-relief-using-dtm-spinal-cord-stimulation-endurance-therapy-301461012.html
SOURCE Medtronic plc