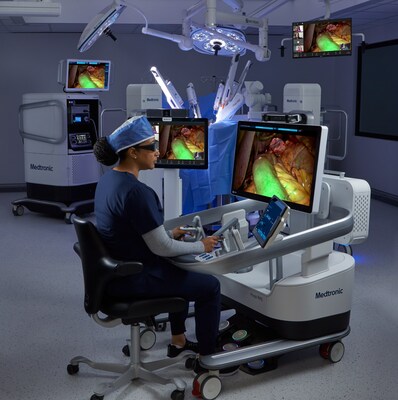
Medtronic initiates U.S. IDE clinical study evaluating Hugo™ robotic-assisted surgery system for gynecological procedures
Medtronic (NYSE: MDT) launched the Embrace Gynecology U.S. IDE clinical study to evaluate the Hugo™ robotic-assisted surgery system for hysterectomy procedures, with enrollment underway and up to 70 patients planned across up to 5 U.S. hospitals. The first total hysterectomy procedures were completed at AHN West Penn Hospital in Pittsburgh on Oct. 8, 2025. Embrace Gynecology is prospective and includes radical, modified radical, and total hysterectomies, inclusive of malignancy cases. This becomes the third U.S. IDE for Hugo RAS after Expand URO and Enable Hernia Repair, both of which met primary safety and effectiveness endpoints. The company notes Hugo RAS is in clinical use in 30+ countries and is CE marked in the EU; in the U.S. the device remains investigational.
Medtronic (NYSE: MDT) ha avviato lo studio clinico IDE statunitense Embrace Gynecology per valutare il sistema di chirurgia assistita da robot Hugo™ per interventi di isterectomia, con arruolamento in corso e fino a 70 pazienti pianificati in al massimo 5 ospedali degli Stati Uniti. Le prime isterectomie totali sono state completate presso l'AHN West Penn Hospital di Pittsburgh l'8 ottobre 2025. Embrace Gynecology è uno studio prospettico e comprende isterectomie radicali, radicali modificati e totali, inclusi casi di malattia. Questo diventa il terzo IDE statunitense per Hugo RAS dopo Expand URO e Enable Hernia Repair, entrambi hanno raggiunto i parametri primari di sicurezza ed efficacia. L'azienda segnala che Hugo RAS è in uso clinico in oltre 30 paesi ed è marcato CE nell'UE; negli Stati Uniti il dispositivo rimane investigazionale.
Medtronic (NYSE: MDT) lanzó el estudio IDE clínico de EE. UU. Embrace Gynecology para evaluar el sistema de cirugía asistida por robot Hugo™ en procedimientos de histerectomía, con reclutamiento en curso y hasta 70 pacientes previstos en hasta 5 hospitales de EE. UU.. Las primeras histerectomías totales se completaron en AHN West Penn Hospital en Pittsburgh el 8 de octubre de 2025. Embrace Gynecology es prospectivo e incluye histerectomías radicales, radicales modificadas y totales, incluyendo casos de malignidad. Esto se convierte en el tercer IDE de EE. UU. para Hugo RAS tras Expand URO y Enable Hernia Repair, ambos que cumplieron los objetivos primarios de seguridad y eficacia. La compañía señala que Hugo RAS está en uso clínico en más de 30 países y está marcado CE en la UE; en EE. UU. el dispositivo permanece en investigación.
메드트로닉(MDT)은 미국 IDE 임상 연구인 Embrace Gynecology를 시작하여 로봇 보조 수술 Hugo™ 시스템을 자궁절제술에 대해 평가하고 있으며, 등록은 진행 중이고 최대 70명의 환자를 목표로 하고, 최대 5개의 미국 병원에서 진행됩니다. 최초의 전체 자궁절제 수술은 2025년 10월 8일 피츠버그의 AHN West Penn Hospital에서 완료되었습니다. Embrace Gynecology는 전향적 연구로, 근치적, 수정된 근치적 및 전체 자궁절제를 포함하며 악성 사례를 포함합니다. 이는 Hugo RAS에 대한 미국 IDE 중 Expand URO와 Enable Hernia Repair에 이어 세 번째가 되며, 두 연구 모두 안전성 및 효과의 주요 목표를 달성했습니다. 회사는 Hugo RAS가 30개국 이상에서 임상 사용 중이며 EU에서 CE 마크를 받았다고 밝혔고, 미국에서는 기기가 아직 연구 중임을 밝히고 있습니다.
Medtronic (NYSE: MDT) a lancé l'étude clinique IDE américaine Embrace Gynecology pour évaluer le système de chirurgie assistée par robot Hugo™ dans les interventions d'hystérectomie, avec un recrutement en cours et jusqu'à 70 patient(e)s prévu(e)s dans jusqu'à 5 hôpitaux américains. Les premières hystérectomies totales ont été réalisées à l'AHN West Penn Hospital de Pittsburgh le 8 octobre 2025. Embrace Gynecology est une étude prospective et comprend des hystérectomies radicales, radicales modifiées et totales, incluant des cas de malignité. Cela devient le troisième IDE américain pour Hugo RAS après Expand URO et Enable Hernia Repair, tous deux ayant atteint les critères principaux de sécurité et d'efficacité. La société indique que Hugo RAS est utilisé cliniquement dans plus de 30 pays et qu'il est marqué CE dans l'UE ; aux États‑Unis, l'appareil reste expérimental.
Medtronic (NYSE: MDT) hat die Embrace Gynecology US-IDE-Studie gestartet, um das Hugo™ roboterunterstützte Operationssystem für Hysterektomie-Verfahren zu evaluieren, mit laufender Rekrutierung und bis zu 70 Patienten geplant in bis zu 5 US-Krankenhäusern. Die ersten totalen Hystektomien wurden am 8. Oktober 2025 im AHN West Penn Hospital in Pittsburgh abgeschlossen. Embrace Gynecology ist prospektiv und umfasst radikale, modifizierte radikale und totale Hystekte, einschließlich malignöser Fälle. Dies wird das dritte US-IDE für Hugo RAS nach Expand URO und Enable Hernia Repair, die beide primäre Sicherheits- und Wirksamkeitsendpunkte erfüllt haben. Das Unternehmen weist darauf hin, dass Hugo RAS in über 30 Ländern klinisch eingesetzt wird und in der EU CE-gekennzeichnet ist; in den USA bleibt das Gerät Untersuchungsgegenstand.
عِلم ميْدترونيك (NYSE: MDT) أطلق دراسة IDE الأمريكية Embrace Gynecology لتقييم نظام Hugo™ للجراحة المدعومة بالروبوت في إجراءات استئصال الرحم، مع تسجيل جارٍ وحتى تخطيط لـ 70 مريضا عبر حتى 5 مستشفيات في الولايات المتحدة. تم إكمال أول عمليات استئصال رحم كلي في مستشفى AHN West Penn في بيتسبرغ في 8 أكتوبر 2025. Embrace Gynecology هي دراسة مستقبلية وتشمل استئصال الرحم الجذري، والجذري المعدل، والكلي، بما في ذلك الحالات الخبيثة. وهذا يصبح IDE الأمريكي الثالث لـ Hugo RAS بعد Expand URO و Enable Hernia Repair، وكلاهما حقق أهداف السلامة والفعالية الأساسية. تشير الشركة إلى أن Hugo RAS مُستخدم سريريًا في أكثر من 30 دولة ومُصنّف CE في الاتحاد الأوروبي؛ أما في الولايات المتحدة فالجهاز لا يزال قيد البحث.
Medtronic(NYSE: MDT) 启动了 Embrace Gynecology 美国 IDE 临床研究,以评估 Hugo™ 机器人辅助手术系统在子宫切除术中的应用,注册正在进行中,计划在最多 5 家美国医院 中纳入最多 70 名患者。首例全子宫切除手术于2025年10月8日在匹兹堡的 AHN West Penn Hospital 完成。Embrace Gynecology 为前瞻性研究,包含根治性、修订根治性和全子宫切除术,以及恶性病例。此成为 Hugo RAS 在美国的第三个 IDE,继 Expand URO 与 Enable Hernia Repair 之后,两者均达到安全性与有效性主要终点。公司表示 Hugo RAS 已在全球 30 多个国家开展临床使用,并在欧盟获得 CE 标记;在美国该设备仍处于研究阶段。
- First U.S. hysterectomy procedures successfully completed on Oct. 8, 2025
- Embrace Gynecology IDE to enroll up to 70 patients across up to 5 U.S. hospitals
- Third U.S. IDE for Hugo RAS after two IDEs that met primary endpoints
- Hugo RAS in clinical use in more than 30 countries with nearly 300 publications
- Hugo RAS holds CE mark in the EU
- Hugo RAS is an investigational device in the U.S. and not for sale
- Embrace study enrollment capped at a modest 70 patients
- U.S. regulatory clearance pending; first U.S. submission under FDA review
Insights
Medtronic launched a U.S. IDE for Hugo RAS in gynecology; first hysterectomies done and enrollment underway across up to five sites.
Medtronic initiated the Embrace Gynecology IDE study on
Key dependencies and risks include patient enrollment pace, site selection quality, and the FDA review timeline for the pending urology submission expected later in the company’s current fiscal year. The study’s data must demonstrate safety and effectiveness across benign and malignant indications to support a U.S. label extension. Monitor enrollment milestones, interim safety signals, and the timing/outcome of the ongoing FDA submission over the next 6–12 months for material regulatory readthroughs.
First procedures completed and enrollment underway in Embrace Gynecology study, furthering the company's progress to expand minimally invasive treatment options to patients in
GALWAY,
The first procedures, total hysterectomies, were successfully completed at AHN West Penn Hospital in
Embrace Gynecology is a prospective multicenter study to evaluate the safety and effectiveness of the Hugo RAS system when used during hysterectomy procedures (radical, modified radical, or total hysterectomies), inclusive of those being treated for malignancies. The study will enroll up to 70 patients across up to 5 U.S. hospitals.
"We are excited to initiate this important clinical study, which aims to investigate surgical treatment options for women in the
The National Cancer Institute estimates that nearly 111,000
"The study name, Embrace, reflects our deeply felt compassion and care for patients and our commitment to providing access to less invasive treatment options for women," said Dr. James Porter, chief medical officer of Robotic Surgical Technologies and Digital Technologies within the Surgical business of Medtronic. "We are grateful for the strong partnership with clinical teams at our study sites and share their excitement about this rigorous scientific study that is helping to usher in a new era of choice for patients in the
Embrace Gynecology becomes the third IDE clinical study for Hugo RAS in the
Also, in July, positive results from a Medtronic-sponsored prospective study of the Hugo RAS system in benign gynecologic procedures outside the
The company's first
Born out of Medtronic's 75-year commitment to expanding access to minimally invasive surgical options worldwide in partnership with clinicians, the Hugo RAS system is currently in clinical use in more than 30 countries on five continents, with nearly 300 independent publications to date.
The Medtronic Hugo RAS system is commercially available in certain geographies. Regulatory requirements of individual countries and regions will determine approval, clearance, or market availability. In the EU, the Hugo RAS system is CE marked. In the
For more information, visit medtronic.com/hugo.
About Medtronic
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Galway,
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
Contacts:
Gary Jeanfaivre
Public Relations
+1-203-556-0777
Ryan Weispfenning
Investor Relations
+1-612-839-4549
1
https://www.aacr.org/patients-caregivers/awareness-months/gynecologic-cancer-awareness-month/
2
https://www.mayoclinic.org/diseases-conditions/endometrial-cancer/diagnosis-treatment/drc-20352466
3 Fitch K, Engel T, Bochner A. Cost differences between open and minimally invasive surgery. Managed Care. 2015;24(9):40–48.
4 Tiwari MM, Reynoso JF, High R, Tsang AW, Oleynikov D. Safety, efficacy, and cost effectiveness of common laparoscopic procedures. Surg Endosc. 2011;25(4):1127-1135.
5 Roumm AR, Pizzi L, Goldfarb NI, Cohn H. Minimally invasive: minimally reimbursed? An examination of six laparoscopic surgical procedures. Surg Innov. 2005;12(3):261–287.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/medtronic-initiates-us-ide-clinical-study-evaluating-hugo-robotic-assisted-surgery-system-for-gynecological-procedures-302577652.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/medtronic-initiates-us-ide-clinical-study-evaluating-hugo-robotic-assisted-surgery-system-for-gynecological-procedures-302577652.html
SOURCE Medtronic plc