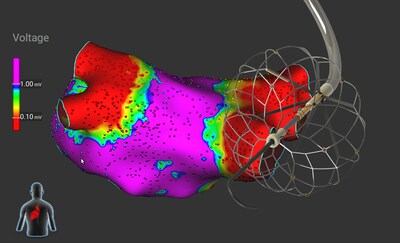
The Medtronic Sphere-360™ Pulse Field Ablation (PFA) catheter, a new paradigm in single-shot ablation, demonstrates impressive results in treating paroxysmal atrial fibrillation
EHRA late-breaking data: Results highlight efficacy, safety, and durability of the novel PFA catheter that is fully integrated with Affera™ Mapping and Ablation System
DUBLIN and
The Sphere-360 catheter was developed with the goal to simplify the atrial fibrillation procedure while enhancing efficiency and providing high durability of lesions. Its unique design includes a large, tissue-conformable lattice tip for efficient energy delivery, with no need to rotate the catheter multiple times in one location as the entire lattice tip delivers PF energy. Furthermore, the catheter is fully integrated with the Affera™ Mapping and Ablation System for complete visualization inside the heart and electroanatomical mapping, making it a true all-in-one single-shot catheter for mapping, ablation, and validation.
The Sphere-360 study, a prospective, single arm, multi-center trial performed in three European centers with a total of 85 patients, demonstrated
A sub-study of patients treated with the optimized pulse underwent remapping procedures;
The study also showed the catheter to have a highly favorable safety profile, with zero incidences of primary safety adverse events including esophageal events, pulmonary vein stenosis, phrenic nerve injury or cardiac tamponade.
"Electrophysiologists are seeking innovative catheter options to address the varied cardiac anatomies encountered in patients, and as the population with atrial fibrillation continues to rise, catheter versatility will become more important than ever to achieve the most favorable outcomes," said Vivek Reddy, M.D., Director of Cardiac Arrhythmia Services, Icahn School of Medicine at Mount Sinai and primary investigator of the Sphere-360 trial. "These results are very encouraging as they show the Sphere-360 catheter has the potential to be an important part of next generation AFib care."
The Sphere-360 PFA catheter includes the following:
- Uniform and efficient PF energy delivery through the entire 34mm conformable lattice tip
- Fully integrated with the Affera Mapping and Ablation System, enabling low to zero fluoroscopy procedure
- Seamlessly adjusts to various shapes to accommodate different PV anatomies
- Real-time local impedance information to assess catheter proximity to tissue
- Over-the-wire design for ease of positioning in the vein and streamlined workflow
- Compatible with a small 8.5Fr sheath
"We have a vision to continually innovate and bring the best technology to AFib patients around the world, driven by our unwavering commitment to maximizing the safety and efficacy of our products," said Khaldoun Tarakji, M.D., MPH, vice president, chief medical officer, Cardiac Ablation Solutions business, which is part of the Cardiovascular Portfolio at Medtronic. "We are thrilled that the Sphere-360 study results validate our dedication to this goal: an all-in-one single shot mapping and pulsed field ablation catheter, that is fully integrated with the Affera Mapping and Ablation System, with a unique design that can conform to any pulmonary vein anatomy and can be used with an 8.5Fr sheath, single transeptal access and zero exchange. All these features make the procedure efficient and outcomes more predictable. We can't wait for what's next."
AFib is one of the most common and undertreated heart rhythm disorders, affecting more than 60 million people worldwide.1 AFib is a progressive disease, meaning it can become worse over time and can increase the risk of serious complications including heart failure, stroke and increased risk of death.2-5 Antiarrhythmic drug (AAD) therapy has been the current standard first-line treatment but is ineffective at controlling AFib in approximately half of patients treated with drug therapy.6-8
Worldwide, the single-shot Sphere-360 catheter is investigational and not approved for sale or distribution. The Affera Mapping and Ablation System, which includes the focal Sphere-9™ Catheter and Affera Mapping System, received CE Mark approval in March 2023.
* Based on data on optimized pulse sub-group. Total cohort
About Medtronic
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Dublin,
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
- Roth GA, Mensah GA, Johnson CO et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J Am Coll Cardiol 2020;76:2982-3021.
- Miyasaka Y, Barnes ME, Bailey KR, et al. Mortality trends in patients diagnosed with first atrial fibrillation: a 21-year community-based study. J Am Coll Cardiol 2007;49:986-92.
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J 2020.
Wolf PA , Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983-8.- Lubitz SA, Moser C, Sullivan L, et al. Atrial fibrillation patterns and risks of subsequent stroke, heart failure, or death in the community. J Am Heart Assoc 2013;2:e000126
- Wazni OM, Dandamudi G, Sood N, et al. Cryoballoon Ablation as Initial Therapy for Atrial Fibrillation. N Engl J Med. January 28, 2021;384(4):316-324.
- Kuniss M, Pavlovic N, Velagic V, et al. Cryoballoon ablation vs. antiarrhythmic drugs: first-line therapy for patients with paroxysmal atrial fibrillation. Europace. March 17, 2021:euab029.
- Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or Drug Therapy for Initial Treatment of Atrial Fibrillation. N Engl J Med. January 28, 2021;384(4):305-315.
Contacts: | |
Leslie Williamson | Ryan Weispfenning |
Public Relations | Investor Relations |
+1-612-227-5099 | +1-763-505-4626 |
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/the-medtronic-sphere-360-pulse-field-ablation-pfa-catheter-a-new-paradigm-in-single-shot-ablation-demonstrates-impressive-results-in-treating-paroxysmal-atrial-fibrillation-302109786.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/the-medtronic-sphere-360-pulse-field-ablation-pfa-catheter-a-new-paradigm-in-single-shot-ablation-demonstrates-impressive-results-in-treating-paroxysmal-atrial-fibrillation-302109786.html
SOURCE Medtronic plc