MannKind Announces FDA Approval of Updated Afrezza® Label Providing Starting Dose Guidance when Switching from Multiple Daily Injections (MDI) or Insulin Pump Mealtime Therapy
Rhea-AI Summary
MannKind (Nasdaq: MNKD) announced that the U.S. Food and Drug Administration approved an update to the Afrezza prescribing information on January 26, 2026 to provide starting mealtime dose guidance when switching adult patients from subcutaneous mealtime insulin (multiple daily injections or insulin pump boluses) to inhaled Afrezza.
The label revision includes a conversion table mapping current injected mealtime doses to recommended Afrezza starting doses (e.g., up to 3 units → 4 units; 4–5 → 8 units; 6–7 → 12 units; 8+ → 16 units). The change was supported by modeling and in vivo data from the Dose Optimization study and the INHALE-3 trial showing improved postprandial glucose outcomes with the approved conversion doses.
Positive
- FDA approved updated Afrezza prescribing information on Jan 26, 2026
- Label now includes a clear conversion table for switching from MDI or pump boluses
- Update supported by Dose Optimization modeling and INHALE-3 trial results showing improved postprandial glucose
Negative
- Revised dosing guidance is limited to adult patients only
News Market Reaction
On the day this news was published, MNKD gained 2.20%, reflecting a moderate positive market reaction. This price movement added approximately $36M to the company's valuation, bringing the market cap to $1.68B at that time.
Data tracked by StockTitan Argus on the day of publication.
Key Figures
Market Reality Check
Peers on Argus
MNKD fell 7.61% while key biotech peers were mixed to mildly negative: ARDX (-1.89%), AUPH (-1.76%), VCEL (-3.3%), HRMY (+0.19%). Momentum scanner flagged SRPT up about 4.92%. This points to a largely stock-specific reaction rather than a coordinated sector move.
Historical Context
| Date | Event | Sentiment | Move | Catalyst |
|---|---|---|---|---|
| Jan 08 | Business update | Positive | +2.7% | Outlined 2026 growth drivers, record Q4 revenue and multiple FDA review dates. |
| Dec 23 | Regulatory update | Positive | -1.0% | FUROSCIX pediatric approval, new patents and PDUFA date for ReadyFlow autoinjector. |
| Dec 01 | Clinical/regulatory | Positive | +1.5% | FDA accepted FUROSCIX ReadyFlow sNDA with PDUFA date set and supportive study data. |
| Nov 11 | Conference appearance | Neutral | +0.0% | Jefferies conference presentation announcement with webcast access details. |
| Nov 10 | Trial discontinued | Negative | -3.7% | Phase 3 ICoN-1 trial of MNKD-101 stopped for futility after interim analysis. |
News tied to regulatory or clinical progress has often seen price moves aligned with the news tone, with only occasional divergence on positive updates.
Recent news shows MannKind advancing multiple regulatory and clinical milestones. On Jan 8, 2026, business updates and 2026 growth drivers, including multiple FDA review acceptances, coincided with a +2.74% move. Prior FUROSCIX regulatory progress and patent extensions around late 2025 were generally positive but saw a small -1% reaction on Dec 23, 2025. The discontinuation of the Phase 3 ICoN-1 trial on Nov 10, 2025 led to a -3.73% move, aligning with negative clinical news. Today’s FDA Afrezza label-update approval fits within this sequence of label and indication-expansion milestones.
Market Pulse Summary
This announcement highlights FDA approval of an updated Afrezza label that provides a clear conversion table when switching from multiple daily injections or insulin pumps. The new guidance specifies starting Afrezza doses of 4, 8, 12, or 16 units based on prior injected mealtime insulin. In the broader context of recent FDA acceptances and label initiatives, this update contributes to MannKind’s ongoing effort to refine Afrezza use in adult patients and may be assessed alongside upcoming regulatory milestones.
Key Terms
subcutaneous medical
postprandial glucose medical
in vivo medical
insulin pump medical
glycemic excursions medical
AI-generated analysis. Not financial advice.
- Updated initial conversion table based on clinical trials in adults showing significantly improved mealtime glycemic excursions
DANBURY, Conn. and WESTLAKE VILLAGE, Calif., Jan. 26, 2026 (GLOBE NEWSWIRE) -- MannKind Corporation (Nasdaq: MNKD), a biopharmaceutical company dedicated to transforming chronic disease care through innovative, patient-centric solutions for cardiometabolic and orphan lung diseases, today announced that the U.S. Food and Drug Administration (FDA) has approved an update to the Prescribing Information for Afrezza® (insulin human) Inhalation Powder, revising recommendations for the starting mealtime dosage when patients switch from subcutaneous mealtime insulin regimens.
“We expect that this label update will help support healthcare providers by providing clearer starting dose guidance when transitioning patients to inhaled insulin from subcutaneous mealtime insulin—whether injections or insulin pumps,” said Dr. Kevin Kaiserman, Senior Vice President, Therapeutic Area Head, Diabetes at MannKind Corporation. “We believe this refinement to the label helps support appropriate initiation of therapy while reinforcing Afrezza’s established clinical profile.”
The updated labeling was supported by modeling data and in vivo results from the Dose Optimization study (see graph) and the INHALE-3 trial that demonstrated improved postprandial glucose outcomes following conversion to inhaled insulin using the now-approved conversion dose.
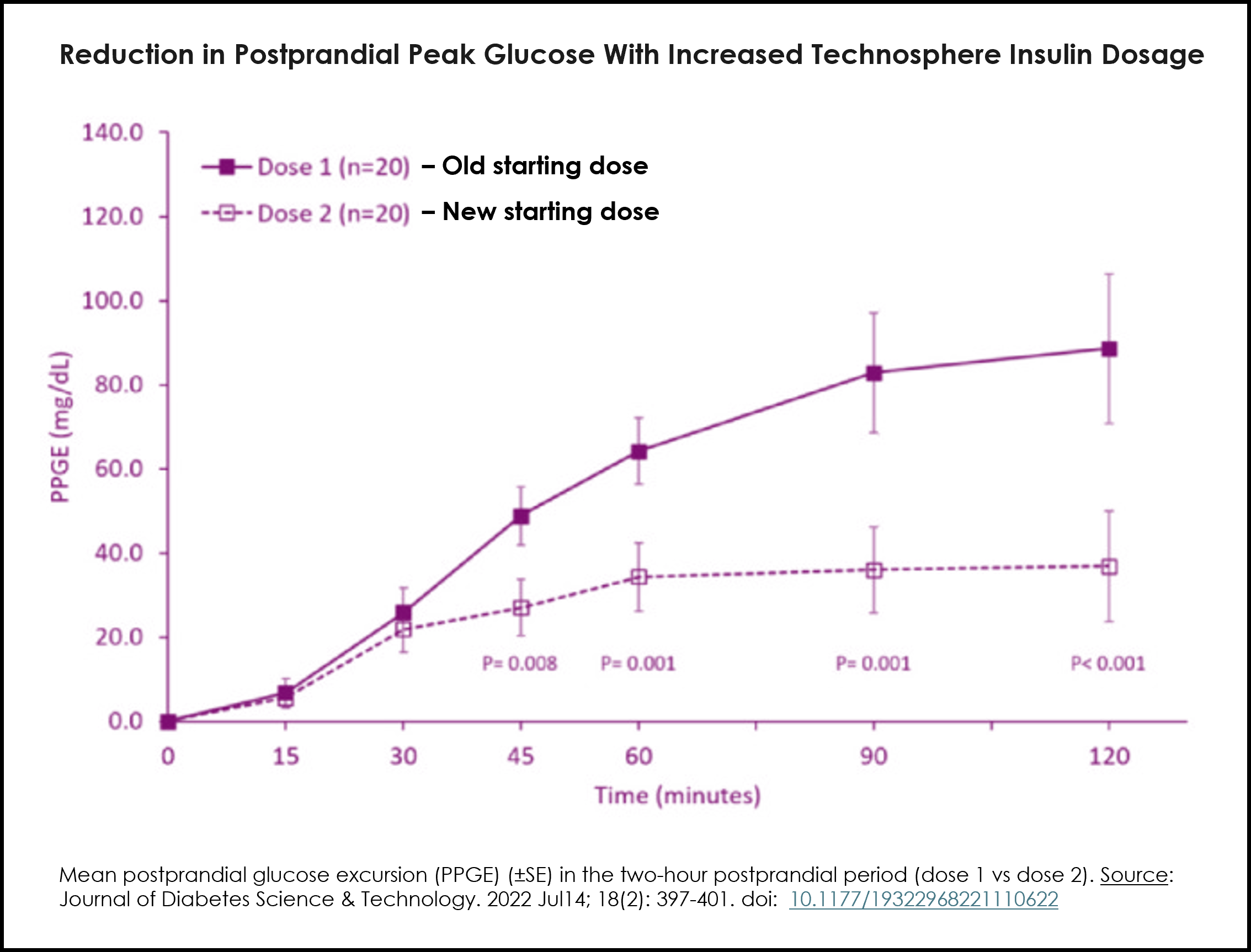
The revised dosing recommendations, provided in the table below, include recommended dose conversions from injected mealtime insulin or insulin pump bolus dosing to the appropriate mealtime dosage of Afrezza and are intended to support a clinically appropriate and safe transition for adult patients initiating Afrezza. Please see the Full Prescribing Information for additional details.
Table 1: Recommended Starting Mealtime Dosage of Afrezza when Switching from Other Mealtime Insulin Regimens
| Current Subcutaneous Mealtime Insulin Dosage | Starting Dosage of AFREZZA |
| Up to 3 units | 4 units |
| 4 to 5 units | 8 units |
| 6 to 7 units | 12 units |
| 8 or more units | 16 units |
About Afrezza
Afrezza® (pronounced uh-frezz-uh) Inhalation Powder is the only ultra rapid-acting inhaled insulin approved by the U.S. Food and Drug Administration to improve glycemic control in adult patients with diabetes mellitus. Administered at the beginning of meals using a small, portable inhaler, Afrezza delivers insulin via MannKind’s proprietary Technosphere® technology, enabling ultra-rapid absorption through the lungs. Afrezza has a fast onset of action and a short duration, more closely mirroring the body’s natural insulin response to meals.
INDICATION AND IMPORTANT SAFETY INFORMATION WITH WARNINGS
Afrezza (insulin human) Inhalation Powder is a rapid-acting inhaled human insulin indicated to improve glycemic control in adults with diabetes mellitus.
Limitations of Use: Not recommended for the treatment of diabetic ketoacidosis or in patients that smoke or have recently stopped smoking.
WARNING: RISK OF ACUTE BRONCHOSPASM IN PATIENTS WITH CHRONIC LUNG DISEASE
- Acute bronchospasm has been observed in Afrezza-treated patients with asthma and chronic obstructive pulmonary disease (COPD)
- Afrezza is contraindicated in patients with chronic lung disease such as asthma or COPD
- Before initiating Afrezza, perform a detailed medical history, physical examination, and spirometry (FEV1) to identify potential lung disease in all patients.
Afrezza is contraindicated: during episodes of hypoglycemia, in patients with chronic lung disease (such as asthma or COPD) because of the risk of acute bronchospasm, and in patients with previous severe hypersensitivity reaction to regular human insulin product or any of the inactive ingredients in Afrezza. Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with Afrezza.
In a study of patients with asthma whose bronchodilators were temporarily withheld for assessment, bronchoconstriction and wheezing following Afrezza dosing was reported. Before initiating therapy, evaluate all patients with a medical history, physical examination, and spirometry (FEV1) to identify potential underlying lung disease. Do not use in patients with chronic lung disease such as asthma or COPD.
Changes in an insulin regimen (e.g., insulin strength, manufacturer, injection site or type, or method of administration) may affect glycemic control and predispose to hypoglycemia or hyperglycemia. If clinically indicated, make any necessary changes to a patient’s insulin regimen under close medical supervision with increased frequency of blood glucose monitoring. For patients with type 2 diabetes, dosage modifications of concomitant oral antidiabetic treatment may be needed.
Hypoglycemia is the most common adverse reaction associated with insulins, including Afrezza. Severe hypoglycemia can cause seizures, may be life-threatening, or cause death. Hypoglycemia can impair concentration ability and reaction time; this may place an individual and others at risk in situations where these abilities are important (e.g., driving or operating other machinery). Hypoglycemia can happen suddenly, and symptoms may differ across patients and change over time in the same patient. Advise patients to recognize and manage hypoglycemia and self-monitor glucose. In patients at higher risk for hypoglycemia and patients who have reduced symptomatic awareness of hypoglycemia, increased frequency of glucose monitoring is recommended.
Afrezza causes a decline in lung pulmonary function over time as measured by FEV1. In clinical trials excluding patients with chronic lung disease and lasting up to 2 years, Afrezza-treated patients experienced a small (40 mL) but greater FEV1 decline than comparator-treated patients. Assess pulmonary function with spirometry at baseline, after the first 6 months of therapy and annually thereafter even in the absence of pulmonary symptoms. In patients who have a decline of ≥
In clinical trials, 2 cases of lung cancer were observed in patients exposed to Afrezza while no cases were reported for the comparators. In both cases, a prior history of heavy tobacco use was identified as a risk factor for lung cancer. Two additional cases of lung cancer (squamous cell and lung blastoma) were reported in non-smokers exposed to Afrezza after the trial completion. These data are insufficient to determine whether Afrezza has an effect on lung or respiratory tract tumors. In patients with active lung cancer, a prior history of lung cancer, or in patients at risk of lung cancer, consider whether the benefits of Afrezza use outweigh this potential risk.
In clinical trials enrolling patients with type 1 diabetes, diabetic ketoacidosis (DKA) was more common in Afrezza-treated patients (
Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with insulin products. If hypersensitivity reactions occur, discontinue Afrezza, treat per standard of care and monitor until symptoms and signs resolve.
All insulin products, including Afrezza, cause a shift in potassium from the extracellular to intracellular space, possibly leading to hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Closely monitor potassium levels in patients at risk of hypokalemia and treat if indicated.
Thiazolidinediones (TZDs), which are peroxisome proliferator-activated receptor (PPAR)-gamma agonists, can cause dose-related fluid retention, particularly when used in combination with insulin. Fluid retention may lead to or exacerbate heart failure. Observe these patients for signs and symptoms of heart failure. If heart failure develops, it should be managed according to current standards of care, and discontinuation or dose reduction of the TZD should be considered.
Most common adverse reactions are hypoglycemia, cough, and throat pain or irritation.
Please see additional Important Safety Information, Full Prescribing Information, including BOXED WARNING, available on Afrezza.com/safety.
About MannKind
MannKind Corporation (Nasdaq: MNKD) is a biopharmaceutical company dedicated to transforming chronic disease care through innovative, patient-centric solutions. Focused on cardiometabolic and orphan lung diseases, we develop and commercialize treatments that address serious unmet medical needs, including diabetes, pulmonary hypertension, and fluid overload in heart failure and chronic kidney disease.
With deep expertise in drug-device combinations, MannKind aims to deliver therapies designed to fit seamlessly into daily life.
Learn more at mannkindcorp.com.
Forward-Looking Statements
Statements in this press release that are not statements of historical fact are forward-looking statements that involve risks and uncertainties. These statements include, without limitation, statements regarding clearer guidance regarding the initiation of insulin therapy. Words such as “believes,” “anticipates,” “plans,” “expects,” “intends,” “will,” “goal,” “potential,” and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based upon MannKind’s current expectations. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, risks associated with developing and commercializing product candidates and other risks detailed in MannKind’s filings with the Securities and Exchange Commission (“SEC”), including under the “Risk Factors” heading of its most recently filed Quarterly Report on Form 10-Q. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this press release. All forward-looking statements are qualified in their entirety by this cautionary statement, and MannKind undertakes no obligation to revise or update any forward-looking statements to reflect events or circumstances after the date of this press release.
AFREZZA, MANNKIND and TECHNOSPHERE are registered trademarks of MannKind Corporation.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/9c8efef3-3fac-4191-b791-45429abb7399

MannKind Contacts: Media Relations: Christie Iacangelo (818) 292-3500 media@mnkd.com Investor Relations: Kate Miranda (781) 301-6869 ir@mnkd.com







