Orocidin’s QR-01 Shows Positive Results in Treating Periodontitis in the treatment of experimental periodontitis in Wistar rats.
Rhea-AI Summary
Nordicus Partners (OTCQB:NORD) subsidiary Orocidin announced positive preclinical results for its lead periodontitis candidate QR-01 on Oct 22, 2025. The announcement reports a second efficacy signal across two animal models (dog and rat) with a rat study run by the ETEP Research Group at the University Complutense of Madrid.
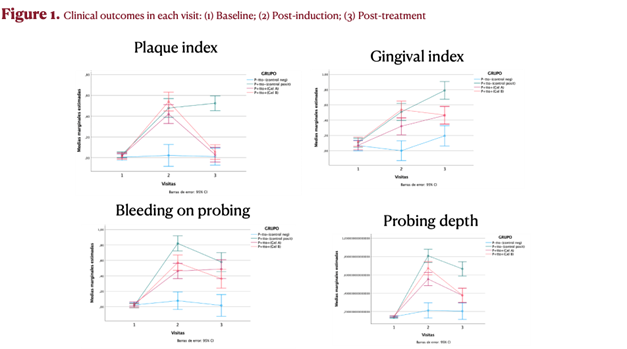
The rat study had two phases: Phase 1 – 3 weeks (periodontitis induction by ligature + bacterial rinses) and Phase 2 – 7 days (periodontal therapy). Four groups were evaluated: negative control, positive control, placebo with periodontal treatment, and QR-01 with periodontal treatment. Key readouts (probing depth, gingival index, bleeding on probing, plaque index) showed statistically significant differences versus negative control, and bleeding on probing decreased in the QR-01 treatment group while it increased in the placebo group.
Positive
- Second positive preclinical result in both dog and rat models
- Study completed with defined protocols: Phase 1 (3 weeks) and Phase 2 (7 days)
- BOP decreased after treatment in the QR-01 group
- PD, GI, BOP, PI showed statistically significant differences versus negative control
Negative
- Results are preclinical (animal studies), not human clinical data
- No quantitative efficacy percentages or human timelines provided
News Market Reaction – NORD
On the day this news was published, NORD declined 7.22%, reflecting a notable negative market reaction.
Data tracked by StockTitan Argus on the day of publication.
BEVERLY HILLS, California, Oct. 22, 2025 (GLOBE NEWSWIRE) -- Orocidin A/S (“Orocidin”), a subsidiary of Nordicus Partners Corporation (OTCQB: NORD) (“Nordicus” or the “Company”), a financial consulting company specializing in supporting Nordic and U.S. life sciences companies in establishing themselves in the U.S. market, today announced the second positive efficacy dog and rat results (in two separate studies) for its lead periodontitis candidate, QR-01, in a preclinical experimental study involving rats with induced periodontitis.
The study was performed by Prof Elena Figuero and Prof Mariano Sanz by the ETEP Research Group at the University Complutense of Madrid, Spain. Professor Figuero and Professor Mariano both acknowledge the value of the study in demonstrating the clinical effect of this compound in improving the clinical outcomes measured in this experimental in vivo investigation, similarly as in the results from the dog study previously performed. “We are both very happy about the data and the outcome of the study” they said.
The rat preclinical in vivo study consisted of two different phases. In Phase 1 (3 weeks), periodontitis was induced using a combination of ligature placement and oral rinses with periodontal pathogenic bacteria. In Phase 2 (7 days), periodontal therapy was performed in the corresponding groups.
These phases led to four experimental groups based on the combination of periodontitis induction and periodontitis treatment, with or without QR-01.
- Negative Control Group: No periodontitis induction and no treatment
- Positive Control Group: Periodontitis induction (ligature placement and oral rinses with bacteria) without periodontal treatment
- Placebo Group: Periodontitis induction (ligature placement and oral rinses with bacteria) with periodontal treatment + placebo gel
- Test Group: Periodontitis induction (ligature placement and rinses with bacteria) with periodontal treatment + QR-01 gel
The results from the rat study demonstrated the following outcome
Probing depth (PD-mm)
- After periodontitis induction, all groups with induced periodontitis exhibited statistically significant differences in PD compared to the negative control group (p < 0.001).
Gingival index (GI)
- After inducing periodontitis, all groups with the condition showed statistically significant differences in GI compared to the negative control group.
Bleeding on probing (BOP)
- After inducing periodontitis, all groups with the condition showed statistically significant differences in BOP compared to the negative control group.
- From a descriptive perspective, after treatment of periodontitis, BOP decreased in the active treatment QR-01 group, whereas an increase was observed in the placebo gel group.
Plaque levels (PI)
- After periodontitis induction, all groups with induced periodontitis showed statistically significant differences in PI compared to the negative control group.
Allan Wehnert, CEO & Founder of Orocidin stated “we are extremely satisfied with the outcome of the study as it confirms the value of QR-01 and shows excellent results. The study was conducted with high professionalism and excellency.”
This is the second pre-clinical animal study demonstrating positive effect in the treatment of periodontitis and shows that the effect of QR-01 is robust and replicable in the treatment of periodontitis and show great prominence for demonstrating clinical effect in periodontitis patients.
About Orocidin
Orocidin’s mission is to develop the preferred treatment against aggressive periodontitis. Our innovative therapeutic agent, QR-01, distinguishes itself through its unique ability to provide treatment of both inflammation and bacterial infection.
About Nordicus Partners Corporation
Nordicus Partners Corporation is the only U.S. publicly traded business accelerator and holding company for Nordic life sciences companies. Leveraging decades of combined management experience in domestic and global corporate sectors, Nordicus excels in corporate finance activities including business and market development, growth strategies, talent acquisition, partnership building, capital raising, and facilitating company acquisitions and sales. In 2024, Nordicus acquired
Cautionary Note Regarding Forward-Looking Statements:
This press release may contain forward-looking statements that involve substantial risks and uncertainties. You can identify these statements by the use of forward-looking terminology such as “may,” “will,” “should,” “expect,” “anticipate,” “project,” “estimate,” “intend,” “continue,” “confident” or “believe” or the negatives thereof or other variations thereon or comparable terminology. You should read statements that contain these words carefully because they discuss our plans, strategies, prospects and expectations concerning our business, operating results, financial condition, prospects of being listed on Nasdaq and other similar matters. We believe that it is important to communicate our future expectations to our investors. There may be events in the future, however, that we are not able to predict accurately or control. Any forward-looking statement made by us in this press release speaks only as of the date on which we make it. Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict all of them. We undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
For further information, contact:
Mr. Henrik Rouf
Chief Executive Officer
hr@nordicuspartners.com
Tel +1 310 666 0750
Attachment








