Insulet Announces CE Mark Approval of Omnipod® 5 Integration with Abbott FreeStyle Libre 2 Plus Sensor
- Omnipod 5 is the world’s first tubeless automated insulin delivery system to achieve CE mark approval with multiple continuous glucose monitoring (CGM) sensor brands.
-
Latest Omnipod 5 integration is expected to be available in the
United Kingdom andNetherlands in the first half of 2024, with additional markets to follow.
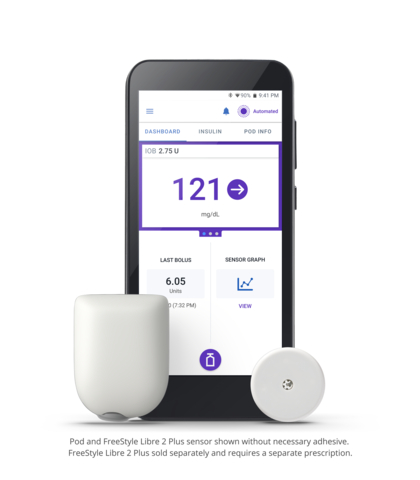
The Omnipod 5 Automated Insulin Delivery System with the Abbott FreeStyle Libre 2 Plus CGM sensor (Photo: Business Wire)
“The addition of the Abbott FreeStyle Libre 2 Plus sensor to our CGM compatibility expands accessibility of Omnipod 5, which has been a priority for Insulet since the system launched in 2022,” said Patrick Crannell, Senior Vice President and International General Manager of Insulet. “We are excited to have achieved this key milestone, which allows us to bring Omnipod 5 and choice of CGM to more people with diabetes.”
Omnipod 5 is the first and only tubeless hybrid closed loop system (also known as automated insulin delivery) that is approved for CE marking and integrated with two CGM sensor brands, Abbott FreeStyle Libre and Dexcom. The Omnipod 5 System consists of the tubeless Pod enhanced with SmartAdjust™ Technology and the Omnipod 5 Controller with its integrated Smartbolus Calculator.
The Pod’s SmartAdjust technology receives a CGM value and trend and predicts where glucose will be 60 minutes into the future. The System corrects every five minutes based on the user’s desired and customized glucose target.
Insulet expects Omnipod 5 integration with the Abbott FreeStyle Libre 2 Plus sensor to be available first in the
To learn more about Omnipod 5, visit the Omnipod website.
About Insulet Corporation:
Insulet Corporation (NASDAQ: PODD), headquartered in
Forward-Looking Statement:
This press release may contain forward-looking statements concerning Insulet's expectations, anticipations, intentions, beliefs, or strategies regarding the future. These forward-looking statements are based on its current expectations and beliefs concerning future developments and their potential effects on Insulet. There can be no assurance that future developments affecting Insulet will be those that it has anticipated. These forward-looking statements involve a number of risks, uncertainties (some of which are beyond its control) or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements, and other risks and uncertainties described in its Annual Report on Form 10-K, which was filed with the Securities and Exchange Commission on February 24, 2023 in the section entitled "Risk Factors," and in its other filings from time to time with the Securities and Exchange Commission. Should one or more of these risks or uncertainties materialize, or should any of its assumptions prove incorrect, actual results may vary materially from those projected in these forward-looking statements. Insulet undertakes no obligation to publicly update or revise any forward-looking statements.
©2024 Insulet Corporation. Omnipod and SmartAdjust are trademarks or registered trademarks of Insulet Corporation in
View source version on businesswire.com: https://www.businesswire.com/news/home/20240207666366/en/
Investor Relations:
Deborah R. Gordon
Vice President, Investor Relations
(978) 600-7717
dgordon@insulet.com
Media:
Angela Geryak Wiczek
Senior Director, Corporate Communications
(978) 932-0611
awiczek@insulet.com
Source: Insulet Corporation







