Journal of Clinical Nuclear Medicine Publishes Results of Tiziana’s Nasal Foralumab in Study Treating Moderate Alzheimer's Disease
- First published report of an AD patient treated with nasal-foralumab showing positive results
- Significant reduction in neuroinflammation observed through PET imaging
- Advancing to Phase-2a study for mild Alzheimer's Disease
- Targeting an unmet medical need with no approved disease-modifying therapies for moderate AD
- Study limited to a single patient case
- Early-stage research requiring larger, controlled studies for validation
Insights
Tiziana's nasal foralumab shows promising early results in reducing brain inflammation in Alzheimer's, potentially addressing a critical unmet need.
The publication in the Journal of Clinical Nuclear Medicine presents intriguing preliminary evidence for Tiziana's intranasal foralumab in Alzheimer's disease (AD). This single-patient expanded access case demonstrates significant reduction in microglial activation - a key marker of neuroinflammation in AD - following treatment.
The study employed sophisticated 18F-PBR06-PET imaging to visualize and quantify microglial activity before and after treatment. The results show notable decreases in standardized uptake values across multiple brain regions including the precuneus, posterior cingulate, bilateral parietal cortices, and cerebellum - all areas typically affected in Alzheimer's pathology.
From a mechanistic perspective, this supports foralumab's proposed mode of action. As the only fully human anti-CD3 monoclonal antibody in development for intranasal delivery, foralumab appears to successfully modulate T-cell function and reduce central nervous system inflammation, consistent with previous findings in multiple sclerosis patients.
What makes this particularly significant is the complete lack of approved disease-modifying therapies for moderate Alzheimer's. While recent FDA approvals have focused on early-stage disease, the moderate AD population represents a substantial unmet need. The intranasal delivery method also potentially overcomes the blood-brain barrier challenges that have plagued many CNS-targeted therapeutics.
However, appropriate caution is warranted as this represents data from a single patient under expanded access rather than a controlled clinical trial. The upcoming Phase 2a study in mild Alzheimer's will be crucial to validate these preliminary findings in a larger population with proper controls and endpoints.
NEW YORK, May 15, 2025 (GLOBE NEWSWIRE) -- Tiziana Life Sciences, Ltd. (Nasdaq: TLSA) (“Tiziana” or the “Company”), a biotechnology company developing breakthrough immunomodulation therapies with its lead development candidate, intranasal foralumab, a fully human, anti-CD3 monoclonal antibody, today announced the publication of a new study in the Journal of ‘Clinical Nuclear Medicine’ demonstrating that intranasal administration of foralumab significantly dampened microglial activation in a 78-year old patient with moderate Alzheimer's disease (AD) treated as part of a Food and Drug Administration (FDA) expanded access program. The findings, published in the prestigious ‘Clinical Nuclear Medicine’ journal, highlight a promising avenue in the treatment of neurological disorders.
This case represents the first use of 18F-PBR06-PET in a patient with moderate AD dementia and the first published report of an AD patient treated with nasal-foralumab. The study, led by Dr. Tarun Singhal and Dr. Howard Weiner of Brigham and Women’s Hospital, a founding member of the Mass General Brigham healthcare system, utilized advanced PET imaging with the [F-18]PBR06 tracer to assess microglial activation before and after treatment. The findings revealed a notable reduction in neuroinflammation following intranasal foralumab administration.
Figure 1.
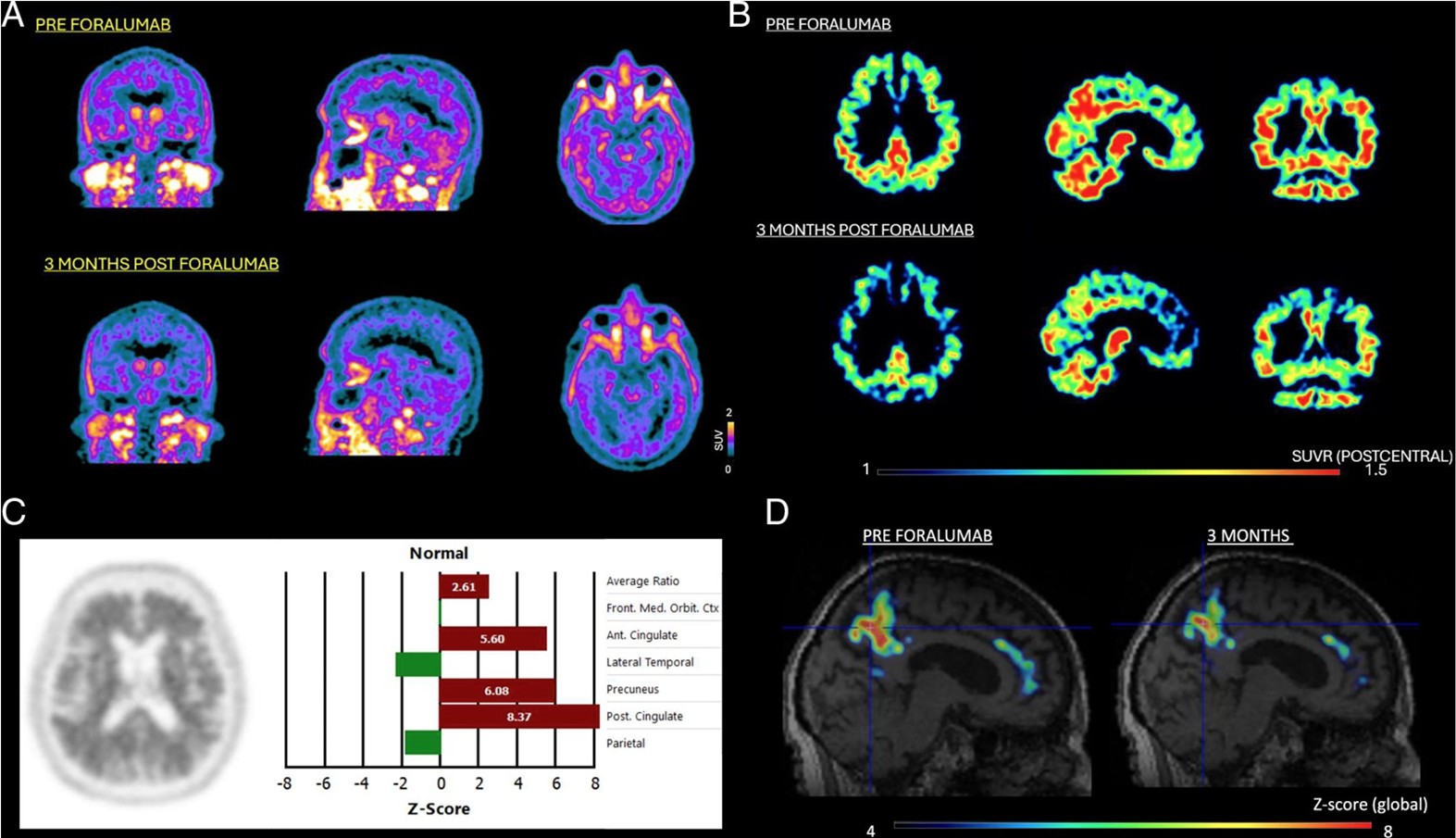
Image Source: Singhal T et al. Dampening of Microglial Activation with Nasal Foralumab Administration in Moderate Alzheimer’s Disease Dementia. Clinical Nuclear Medicine 2025 May 13. doi: 10.1097/RLU.0000000000005955. Online ahead of print.
A, 18F-PBR06-PET shows significant decreased standardized uptake value (SUV) after 3 months of treatment (bottom row) with nasal-foralumab as compared to baseline (top row).
B, SUV-ratio (SUVR) images demonstrate a decrease in PET signal following treatment, particularly in bilateral precuneus, posterior cingulate, bilateral parietal cortices and cerebellum (bottom row) as compared with pre-treatment baseline (top row).
C, A baseline amyloid-positive 18F-Florbetapir-PET scan in the patient
D, z-score maps of 18F-PBR06-PET focusing on the precuneus, posterior cingulate, and anterior cingulate regions show abnormally increased microglial activation in these regions (left) before treatment, which decreased following treatment with nasal-foralumab (right).
“The marked decrease in microglial PET signal following nasal-foralumab treatment provides preliminary evidence that intranasal foralumab can modulate microglial activity in Alzheimer's disease. This supports the potential efficacy of nasal foralumab in targeting microglial activation in AD,” said Tarun Singhal, M.B.B.S., M.D., Associate Professor of Neurology at Harvard Medical School, Director of the PET Imaging Program in Neurologic Diseases, and Associate neurologist and nuclear medicine physician at Brigham and Women’s Hospital. “Given the role of microglia in neurodegeneration, these results are encouraging and warrant further investigation in larger, controlled studies with additional quantitative approaches.”
“These findings from Dr. Singhal et al, are consistent with our previously reported effects of nasal- foralumab in decreasing microglial activation in non-active secondary progressive multiple sclerosis patients,” Commented Ivor Elrifi, CEO of Tiziana Life Sciences. “There are currently no approved disease-modifying therapies for moderate Alzheimer’s Disease, and it is clearly an area of unmet need. A phase-2a study of nasal-foralumab in mild Alzheimer’s is being initiated.”
Foralumab is the only fully human anti-CD3 monoclonal antibody in clinical development for intranasal delivery. By modulating T cell function, it aims to reduce inflammation in the central nervous system. Previous studies have shown its potential in multiple sclerosis and other neuroinflammatory conditions.
The full study is available online in Clinical Nuclear Medicine at: https://journals.lww.com/nuclearmed/Fulltext/9900/dampening_of_microglial_activation_with_nasal.1721.aspx.
About Foralumab
Foralumab, a fully human anti-CD3 monoclonal antibody, is a biological drug candidate that has been shown to stimulate T regulatory cells when dosed intranasally. At present, 10 patients with Non-Active Secondary Progressive Multiple Sclerosis (na-SPMS) have been dosed in an open-label intermediate sized Expanded Access (EA) Program (NCT06802328) with either an improvement or stability of disease seen within 6 months in all patients. In addition, intranasal foralumab is currently being studied in a Phase 2a, randomized, double-blind, placebo-controlled, multicenter, dose-ranging trial in patients with non-active secondary progressive multiple sclerosis (NCT06292923).
Foralumab is the only fully human anti-CD3 monoclonal antibody (mAb) currently in clinical development. The non-active SPMS intranasal foralumab Phase 2 trial (NCT06292923) began screening patients in November of 2023. Immunomodulation by intranasal foralumab represents a novel avenue for the treatment of neuroinflammatory and neurodegenerative human diseases.[1],[2]
About Tiziana Life Sciences
Tiziana Life Sciences is a clinical-stage biopharmaceutical company developing breakthrough therapies using transformational drug delivery technologies to enable alternative routes of immunotherapy. Tiziana’s innovative nasal approach has the potential to provide an improvement in efficacy as well as safety and tolerability compared to intravenous (IV) delivery. Tiziana’s lead candidate, intranasal foralumab, which is the only fully human anti-CD3 mAb currently in clinical development, has demonstrated a favorable safety profile and clinical response in patients in studies to date. Tiziana’s technology for alternative routes of immunotherapy has been patented with several applications pending and is expected to allow for broad pipeline applications.
For more information about Tiziana Life Sciences and its innovative pipeline of therapies, please visit www.tizianalifesciences.com.
Forward-Looking Statements
Certain statements made in this announcement are forward-looking statements. These forward-looking statements are not historical facts but rather are based on the Company's current expectations, estimates, and projections about its industry, its beliefs, and assumptions. Words such as 'anticipates,' 'expects,' 'intends,' 'plans,' 'believes,' 'seeks,' 'estimates,' and similar expressions are intended to identify forward-looking statements. These statements are not guarantees of future performance and are subject to known and unknown risks, uncertainties, and other factors, some of which are beyond the Company's control, are difficult to predict, and could cause actual results to differ materially from those expressed or forecasted in the forward-looking statements. The Company cautions security holders and prospective security holders not to place undue reliance on these forward-looking statements, which reflect the view of the Company only as of the date of this announcement. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including: the uncertainties related to market conditions and other factors described more fully in the section entitled ‘Risk Factors’ in Tiziana’s Annual Report on Form 20-F for the year ended December 31, 2024, and other periodic reports filed with the Securities and Exchange Commission. The forward-looking statements made in this announcement relate only to events as of the date on which the statements are made. The Company will not undertake any obligation to release publicly any revisions or updates to these forward-looking statements to reflect events, circumstances, or unanticipated events occurring after the date of this announcement except as required by law or by any appropriate regulatory authority.
For further inquiries:
Tiziana Life Sciences Ltd
Paul Spencer, Business Development, and Investor Relations
+44 (0) 207 495 2379
email: info@tizianalifesciences.com
[1] https://www.pnas.org/doi/10.1073/pnas.2220272120
[2] https://www.pnas.org/doi/10.1073/pnas.2309221120
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/cd486638-7caa-40f2-8c97-1c9c48a3ea9e








